Caco-2-based P-gp Substrate Assessment Service
P-gp Affects Drug Absorption and Resistance
P-glycoprotein (P-gp) belongs to the ABC (ATP-binding cassette) superfamily. Specifically, it is classified under the ABCB subfamily within the ABC transporters. P-gp is an important drug transporter that can efflux a wide range of clinically relevant hydrophobic drugs out of cells through ATP hydrolysis, thereby affecting the bioavailability and distribution of drugs.
P-gp is abundantly present on the apical surface of enterocytes in the small intestine, functioning to facilitate oral drug absorption. It is also expressed at several important biological barriers, protecting the body from toxic insults but potentially blocking the entry of useful therapeutics. The structural transition of P-gp between different conformations reflects its conformational flexibility and broad substrate specificity.
Caco-2-based P-gp Substrate Assessment
Orally administered drugs must traverse the intestinal epithelial barrier to be effectively absorbed. A vital feature influencing the rate and extent of absorption in vivo is intestinal permeability, which directly affects the bioavailability of potential drug candidates. The Caco-2 cell line can mimic the structure of the human intestinal epithelium and is derived from human colon adenocarcinoma cells. This cell line has become a popular in vitro system for assessing drug transport and evaluating the permeability of new compounds.
Creative Biolabs offers P-gp substrate assessment services based on Caco-2 cells by HPLC-MS. The purpose of this assay is to determine if the test compound acts as a substrate for the P-glycoprotein efflux transporter found in Caco-2 cells. The evaluation of the test compound's permeability is conducted in both apical-to-basolateral (A-B) and basolateral-to-apical (B-A) directions, with additional tests performed with a P-gp-specific inhibitor, verapamil.
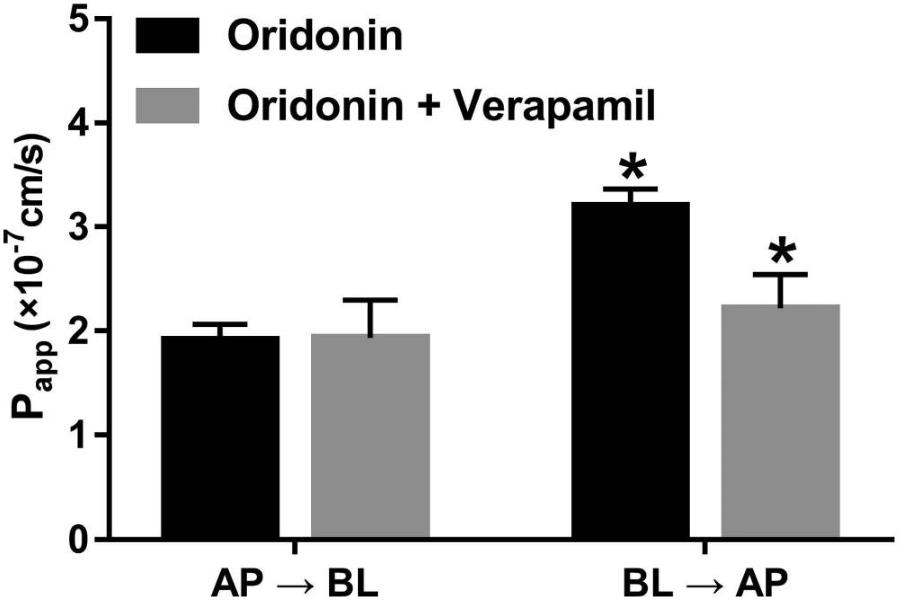 Fig.1 Verapamil inhibits the efflux of oridonin (a typical P-gp substrate).1,2
Fig.1 Verapamil inhibits the efflux of oridonin (a typical P-gp substrate).1,2
Caco-2 cells can be employed in various studies, including drug-drug interaction assessments, evaluation of nutrient absorption, and investigation of transport mechanisms for both hydrophilic and lipophilic compounds. Using the Caco-2 cell line in P-gp substrate assessment offers several advantages:
-
Physiological Relevance: Caco-2 cells differentiate in culture to form tight junctions and exhibit characteristics similar to the epithelial lining of the human small intestine, making them a relevant model for studying intestinal absorption.
-
Universality: The Caco-2 cells are widely utilized to assess the transport properties of compounds, providing insights into how drugs may be absorbed in the gastrointestinal tract.
Human Intestinal Absorption (HIA) refers to the ability of a drug to move from the gastrointestinal tract into the bloodstream. This metric is essential for forecasting a drug's bioavailability, which ultimately influences its effectiveness in treatment. Caco-2-based P-gp substrate assessment is of significance for the research on HIA. The assessment may help you determine which medications can be effectively administered orally and which ones might need different delivery approaches.
Creative Biolabs is willing to offer a variety of services in ADME to meet your needs. Contact us to know more details about P-gp substrate assessment. You may also interested in:
References
-
Liu, J., et al. "Influence of verapamil on the pharmacokinetics of oridonin in rats." Pharmaceutical biology 57.1 (2019): 787-791.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 Verapamil inhibits the efflux of oridonin (a typical P-gp substrate).1,2
Fig.1 Verapamil inhibits the efflux of oridonin (a typical P-gp substrate).1,2
 Download our brochure
Download our brochure