In Vitro Cytochrome P450 2C19 (CYP2C19) Intrinsic Clearance Measurement Service
CYP2C19 Intrinsic Clearance Studies
Cytochrome P450 2C19 (CYP2C19), a member of the CYP2C subfamily, is an enzyme that is mainly found in the liver. CYP2C19 is involved in the metabolism of a large number of medications, including proton pump inhibitors, certain antidepressants, and antiplatelet medications like clopidogrel.
The CYP2C19 phenotyping assay is a valuable tool used to assess the function of the CYP2C19 enzyme in the metabolic clearance of drugs. There are several methods to carry out a CYP2C19 phenotyping assay, with one common approach being the use of recombinant enzyme systems. Recombinant CYP2C19 enzyme can be expressed and isolated for in vitro studies to evaluate its activity towards the test drug substrate. This allows for a controlled environment to determine the enzyme kinetics and metabolic pathways involved.
Intrinsic clearance measurement is an important aspect of drug discovery and development, as it helps predict the rate at which a drug is metabolized and eliminated from the body. At Creative Biolabs, our CYP2C19 phenotyping assay using recombinant enzyme systems offers a reliable and robust method to evaluate the involvement of CYP2C19 in drug metabolism. This assay allows for the determination of the intrinsic clearance of the drug, providing valuable information on its clearance rate and stability. Our comprehensive assay services are tailored to meet the specific needs of our clients, ensuring accurate and reliable results for drug development and personalized medicine applications.
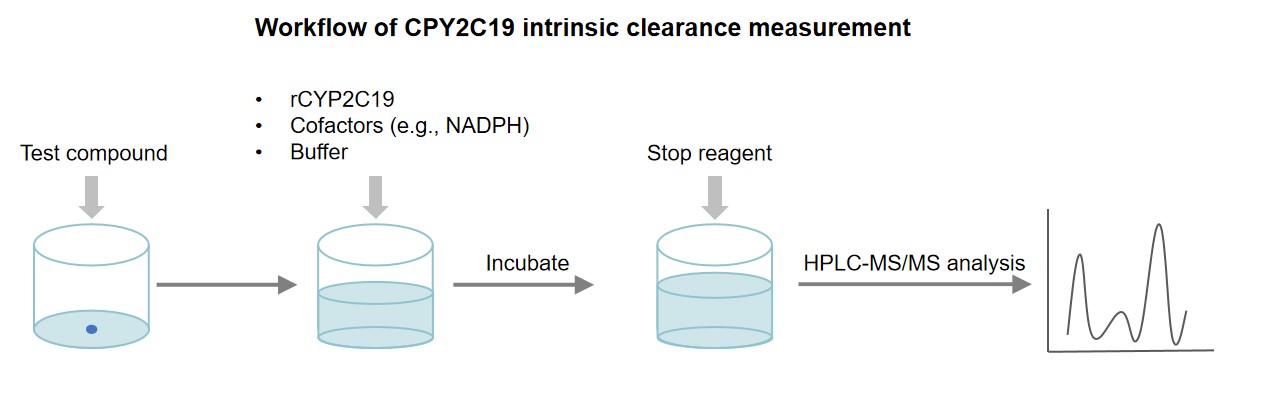
CYP2C19 Intrinsic Clearance Measurement Using Recombinant Human CYP2C19 Enzymes
-
Preparation of assay conditions: Prepare a reaction mixture containing the substrate, cofactors (e.g., NADPH), and the recombinant CYP2C19 enzyme in appropriate buffer conditions.
-
Incubation of test compound with CYP2C19 enzyme: The test compound is incubated with CYP2C19 enzyme reaction mixture at 37°C for several time points.
-
Measurement of parent compound: Terminate the reaction by adding a stop solution or freezing the samples. The reaction mixture is then analyzed to detect and quantify the parent compound using analytical techniques such as HPLC-MS/MS.
-
Determination of intrinsic clearance: From the initial and remaining of the test compound, the intrinsic clearance of the compound by CYP2C19 can be calculated. This parameter provides an estimate of the enzymatic activity of CYP2C19 towards the test compound.
More About Our Services
Our lab aims to offer various Enzyme Reaction Phenotyping Assessment Services. Whether you need to assess the involvement of CYPs in the metabolism of a test drug or evaluate the intrinsic clearance of a compound, we are here to offer customized phenotyping assays to support your research and development needs.
Our ADME services include in vitro ADME studies to measure drug permeability, metabolic stability, protein binding, and potential for drug-drug interactions. By working with Creative Biolabs, you can benefit from our rich experience in performing in vitro studies to measure intrinsic clearance and assess the potential pharmacokinetic profile of your compounds. Our team is equipped with the necessary expertise and resources to provide accurate and reliable results tailored to meet the specific requirements of our clients.
For Research Use Only | Not For Clinical Use



 Download our brochure
Download our brochure