Multidrug And Toxin Extrusion Protein (MATE) Substrate Assessment Service
SLC in Drug Transporters
Drug transporters facilitate the movement of drugs, as well as endogenous molecules and toxins, across cellular membranes. Drug transporters are widely expressed in various tissues, and they significantly influence the absorption, distribution, and excretion of drugs.
MATE (Multidrug And Toxin Extrusion Protein) belongs to the SLC superfamily. Drug transporters can be classified into these main superfamilies: ATP-Binding Cassette (ABC) superfamily, Solute-Linked Carrier (SLC) superfamily, and Solute Carrier Organic Anion (SLCO) superfamily. The SLCO superfamily is responsible for transporting larger molecules, such as bile salts and xenobiotics. Their transport mechanisms are less well understood compared to those of the ABC and SLC superfamilies.
|
|
ABC Transporters
|
SLC Transporters
|
|
Energy Source
|
Utilize the hydrolysis of ATP as their energy source for active transport, allowing them to move substrates unidirectionally across membranes.
|
Primarily rely on electrochemical or concentration gradients for secondary active transport, and do not directly use ATP for energy.
|
|
Transport Mechanism
|
Are generally classified as primary active transporters and can function as uniporters, moving one type of substrate across the membrane.
|
Are often secondary active transporters and can be further categorized into antiporters (transporting substrates in opposite directions), symporters (transporting substrates in the same direction), and uniporters (transporting a single substrate).
|
|
Structural Characteristics
|
Typically have a structure that includes two transmembrane domains and two nucleotide-binding domains.
|
Exhibit a variety of structural folds and can have different numbers of transmembrane helices depending on the specific transporter family.
|
|
Substrate Specificity
|
A broad range of substrates
|
A diverse range of substrates.
However, the substrate specificity can vary widely among the different SLC families.
|
The SLC family consists of 65 different families that are responsible for transporting a diverse array of substrates. SLC transporters are further categorized into subfamilies based on their transport mechanisms and substrate types. The MATE family, also known as SLC47, transporters are involved in the extrusion of a variety of substrates, including many clinically relevant drugs and toxic compounds. They typically transport low molecular weight cationic substrates, but can also handle zwitterionic and ionic molecules.
MATE Substrate Assessment at Creative Biolabs
The MATE family includes several isoforms, such as MATE1 (SLC47A1) and various variants of MATE2 (including MATE2-B and MATE2-K). MATE1 exhibits broad substrate specificity and is significantly expressed in the liver, skeletal muscle, and kidneys. MATE2 is expressed in the kidneys and testes of mice and humans.
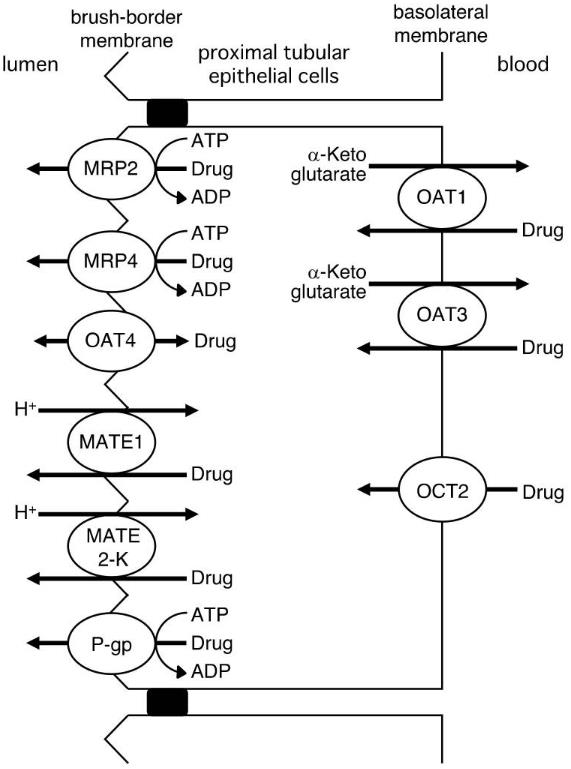 Fig.1 MATE1 and MATE2-K mediating renal tubular secretion drugs.1,2
Fig.1 MATE1 and MATE2-K mediating renal tubular secretion drugs.1,2
Creative Biolabs provides an assessment to evaluate whether test compounds are substrates for MATE transporter (MATE1 or MATE2-K) using LC-MS/MS. To assess specific transporter uptake versus passive permeability in WT control cells, we treat both transporter cell lines and wild-type control cells with compounds. The percentage reduction in test compound uptake in transporter cell lines due to a transporter-specific inhibitor is then quantified.
MATE Substrate Assessment helps determine whether a compound is a substrate for MATE transporters, which is essential for predicting how drugs will be absorbed, distributed, and eliminated in the body. Creative Biolabs is dedicated to helping researchers evaluate the potential for compounds to cause toxicity by assessing MATE substrates, please feel free to contact us. Additionally, you may also be interested in our other services in ADME, especially: Drug-Drug Interactions, Analysis of Protein Binding, and Analysis of Drug Metabolic Stability.
References
-
Uwai Y. " Enantioselective Drug Recognition by Drug Transporters. " Molecules 23.12 (2018): 3062.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only | Not For Clinical Use


 Fig.1 MATE1 and MATE2-K mediating renal tubular secretion drugs.1,2
Fig.1 MATE1 and MATE2-K mediating renal tubular secretion drugs.1,2
 Download our brochure
Download our brochure