- Home
- ADC Development
- ADC In Vitro Analysis
- ADC Biochemical Analysis
ADC Biochemical Analysis Services
Creative Biolabs is a leader in providing services for antibody-drug conjugates (ADCs) development and analysis. With the comprehensive analytical platform and experienced lab personnel, Creative Biolabs offers global clients with an integrated package for ADC biochemical characterization services to meet your novel ADC development goals. This package includes a variety of different modules, including ADC structural analysis, ADC stability analysis, drug to antibody ratio (DAR) evaluation, drug load distribution assessment, as well as linker and conjugation sites analysis.
Compared to traditional anti-tumor drugs, ADCs utilize the specific monoclonal antibodies (mAbs) to achieve targeted delivery of cytotoxic small molecule drugs. The exquisite designs of an ADC can maximize the delivery of antitumor agents to the targeted tissue while minimize its toxic side effects to normal tissues. The three individual components of an ADC: cytotoxic drug, monoclonal antibody, and chemical linker, are indispensable for its overall therapeutic effects and they also contribute significantly to the biochemical characteristics of an ADC.
ADC preparation involves the chemical conjugation of the three components and depending on the conjugation strategy used, this process often yields complex and heterogeneous products. In order to develop a successful ADC, it is important to extrapolate its biophysical and biochemical properties and monitor the whole preparation process by using the appropriate analytical methods. Generally speaking, information regarding the exact structure of an ADC, including the primary structure and higher ordered structure, as well as the drug to antibody ratio (DAR) and drug load distribution, is crucial for the understanding of the ADC chemistry and quality. In the meantime, the stability of the three components under different physical or chemical conditions need to be carefully evaluated to perfect the ADC design, reduce the off-target drug release, and achieve highly specific drug delivery.
With years of accumulative work, Creative Biolabs has established an integrated analytical platform to provide customers with comprehensive services to determine the biophysical and biochemical properties of an ADC. We utilize various state-of-the-art analytical equipment, including UV/Vis, HIC, SDS-PAGE, CE, IEC, ITC, RP-HPLC, LC-MS, ELISA, SPR, flow cytometry…, to measure the ADC structural properties, stability, the drug load properties, and the linker properties.
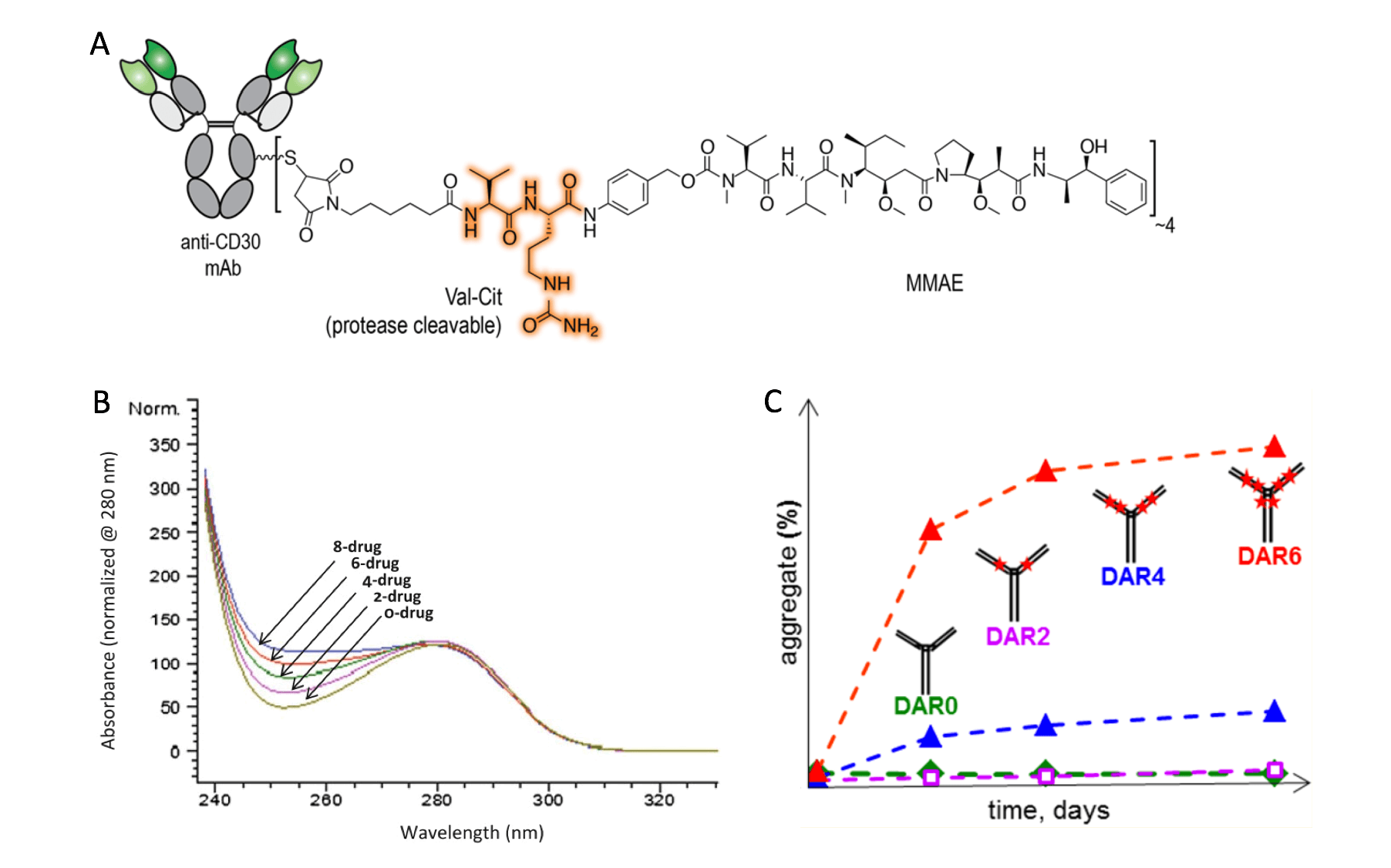 Biophysical and biochemical characterization of an ADC. (A) the structure of Brentuximab vedotin, an ADC formed by conjugation of an anti-CD30 antibody with MMAE via a vc peptide linker to the Cys residues (Chem. Sci., 2016); (B) UV spectra of drug-loaded species and DAR estimation (Antibody drug conjugates., 2013); (C) DAR affects the physical stability of an ADC by promoting aggregation in some cases (Bioconjugate Chem., 2014)
Biophysical and biochemical characterization of an ADC. (A) the structure of Brentuximab vedotin, an ADC formed by conjugation of an anti-CD30 antibody with MMAE via a vc peptide linker to the Cys residues (Chem. Sci., 2016); (B) UV spectra of drug-loaded species and DAR estimation (Antibody drug conjugates., 2013); (C) DAR affects the physical stability of an ADC by promoting aggregation in some cases (Bioconjugate Chem., 2014)
Besides ADC biochemical analysis, Creative Biolabs also offers options services for the evaluation of ADC in vitro efficacy using both regular cell lines and highly innovated 3D cultured cell lines. With our experienced science team and advanced analytical platform, Creative Biolabs is dedicated to provide our global clients with the comprehensive analytical services for ADC development projects. Please contact us for more information and a detailed quote.
References:
- Ouyang, J.; et al. Drug-to-antibody ratio (DAR) and drug load distribution by hydrophobic interaction chromatography and reversed phase high-performance liquid lhromatography. Antibody drug conjugates. 2013.
- Akkapeddi, P.; et al. Construction of homogeneous antibody-drug conjugates using site-selective protein chemistry. Chem. Sci. 2016, 7(5): 2954-2963.
- Adem, Y.T.; et al. Auristatin antibody drug conjugate physical instability and the role of drug payload. Bioconjugate Chem. 2014, 25(4): 656-664.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
