- Home
- ADC Development
- ADC In Vitro Analysis
- ADC In Vitro Efficacy Evaluation
ADC In Vitro Efficacy Evaluation Services
Creative biolabs is a well-recognized leader in antibody-drug conjugates (ADCs) developments and we offer a comprehensive set of services to help our clients with ADC preparation, biochemical analysis, and especially, in vitro efficacy evaluation.
Antibody-drug conjugates are a class of highly innovative targeted antitumor agents formed by the covalent coupling of small molecular cytotoxic drugs (payloads) to monoclonal antibodies via small chemical linkers. The delicate design of an ADC empowers it with a series of advantages comparing to conventional cancer therapies, including improved payload efficacy, enhanced targeting accuracy, and reduced collateral damage to normal tissues. By design, the major toxicity of an ADC comes from the loaded toxic drug, since the general mode of action of ADC includes the target binding via antibody mediated immuno-recognition, internalization by receptor mediated endocytosis, payload release through lysosomal degradation, and payload mediated cellular pathway disruption. In the meantime, the Fc portion of the antibody can also exert effector activities, contributing additional toxicity. Thus, as a newly designed molecule, the efficacy of an ADC should be reevaluated.
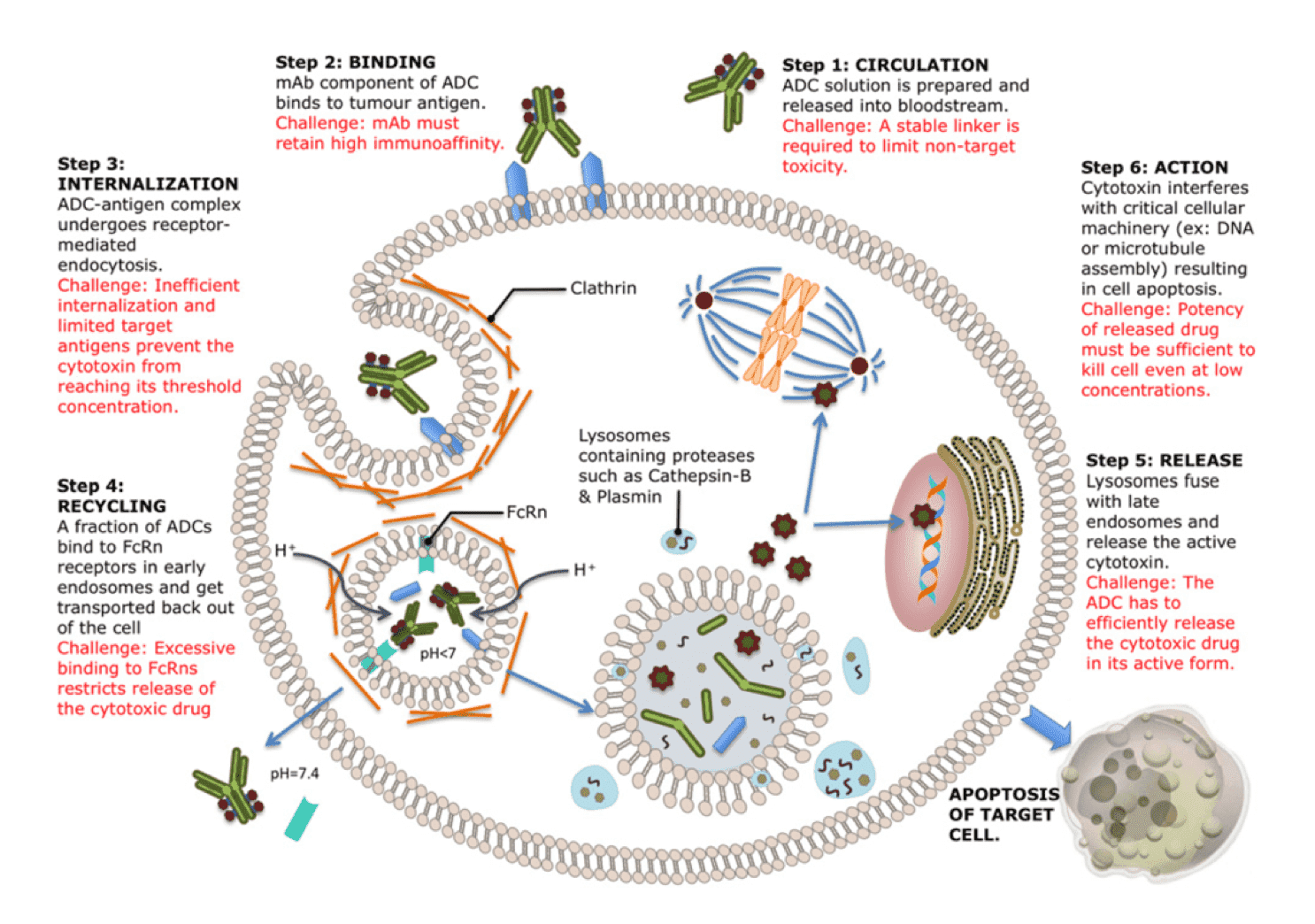 ADC mode of action and challenges associated with ADC designs. The toxic payloads on an ADC are released after internalization. As novel therapeutics, the successful design of an ADC must meet the criteria of tight binding, stable in circulation, internalization upon antigen association… (Bioscience Rep., 2015)
ADC mode of action and challenges associated with ADC designs. The toxic payloads on an ADC are released after internalization. As novel therapeutics, the successful design of an ADC must meet the criteria of tight binding, stable in circulation, internalization upon antigen association… (Bioscience Rep., 2015)
In vitro efficacy evaluation of an ADC provides important information to guide the improvement of the ADC design and the planning of the downstream in vivo evaluation studies. Besides the obvious in vitro cytotoxicity evaluation, another three aspects of an ADC are extensively assessed to elucidate the in vitro efficacy of such delegate molecule:
- Antigen binding affinity—the antigen binding affinity of the newly designed ADCs must be reevaluated to ensure that they retain similar tumor surface antigen binding affinities as that of the corresponding unconjugated monoclonal antibodies.
- Internalization—since internalization is a prerequisite for ADC mode of action, an ADC should show rapid and sufficient internalization into tumor cells and correct transportation to appropriate intracellular compartments to release the payloads.
- Fc mediated toxicity—the major function of the antibody portion in an ADC is to serve as a guidance system for targeted drug delivery. However, they may also elicit effector activity by the antibody Fc region to achieve effector cell/factor recruitment and destroy tumor cells. The Fc-mediated immune response adds a “secondary efficacy” to an ADC and this feature should also be evaluated.
Creative biolabs has established a comprehensive analytical platform to provide a variety of in vitro chemical or cellular assay methods to evaluate the in vitro efficacy/potency of an ADC. Examples of analytical equipment and stepwise services include (not limited to):
- ADC affinity measurements
- Surface plasmon resonance (SPR)
- Enzyme-linked immunosorbent assay (ELISA)
- Fluorescence-activated cell sorting (FACS) flow cytometry
- ADC Internalization detection
- Radiolabels
- Fluorescent microscopy
- Flow cytometry
- ADC In vitro cytotoxicity analysis
- Cell viability
- Apoptosis
- Cell-cycle
- ADC Fc cytotoxicity
- Antibody dependent cellular cytotoxicity (ADCC)
- Complement dependent cytotoxicity (CDC)
- Antibody dependent cellular phagocytosis (ADCP)
After years of pioneering work in ADC development and manufacture, Creative biolabs has established an integrated platform for ADC preparation, including antibody discovery and production, organic chemistry synthesis, biochemical analysis and pharmacological evaluation. We are committed to provide clients with top-quality in vitro ADC efficacy evaluation, ADC biochemical analysis services, and highly innovated 3D cultured cell lines for ADC efficacy evaluation mimicking solid tumor. Please contact us for more information and a detailed quote.
Reference:
- Jackson, D.; et al. In vitro and in vivo evaluation of cysteine and site specific conjugated herceptin antibody-drug conjugates. PLOS One. 2014, 9(1): e83865.
- Peters, C.; et al. Antibody–drug conjugates as novel anti-cancer chemotherapeutics. Bioscience Rep. 2015, 35(4): e00225.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
