- Home
- UTC Development
- Bispecific ADC Development
- Fast-internalizing Receptor based Bispecific ADC Development
- c-Met based Bispecific ADC Development
c-Met based Bispecific ADC Development Service
Bispecific antibody-drug conjugates (ADCs) have already demonstrated benefits for the treatment of cancer in several preclinical studies, showing improved drug selectivity and efficacy. As a professional service provider in ADC production for years, Creative Biolabs has successfully completed a significant number of ADC development projects. Our customers, ranging from research institutions to large biotech companies, have taken full advantage of our ADC platforms. Creative Biolabs now offers a comprehensive set of c-Met-based bispecific ADCs development services for our worldwide clients.
Background
The Overview of c-Met
c-Met is a proto-oncogene that encodes a protein known as hepatocyte growth factor receptor (HGFR). The ligand of c-Met, hepatocyte growth factor (HGF) was identified as a potent mitogen/morphogen. The HGF/c-Met signaling pathway has been demonstrated to play important roles in the development and progression of various cancers. HGF/c-Met signaling through downstream effectors stimulates diverse cellular processes such as cell proliferation, differentiation, migration, invasion, and survival. c-Met has become a promising therapeutic target in the treatment of cancer.
c-Met is produced as a single-chain precursor and processed to yield a mature receptor composed of a glycosylated, extracellular alpha subunit disulfide-bonded to a transmembrane beta subunit. The extracellular portion of c-Met is composed of a Sema domain, a cysteine-rich, Met-related-sequence domain, and four immunoglobulin (Ig)-like modules responsible for binding HGF. The intracellular portion of c-Met is composed of a juxtamembrane domain, a tyrosine kinase domain, and a C-terminal regulatory tail responsible for signal transduction. Tyrosine residues in the tyrosine kinase domain regulate the kinase activity of c-Met, and tyrosine residues in the C-terminal regulatory tail are important for the recruitment of downstream adapters.
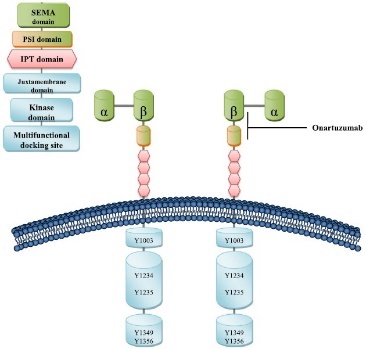 Fig.1 Structure of c-Met and binding sites for c-Met monoclonal antibody.1
Fig.1 Structure of c-Met and binding sites for c-Met monoclonal antibody.1
Antibodies-based Therapeutics Targeting c-Met
Multiple therapeutic antibodies targeting c-Met are currently in preclinical and clinical development. Onartuzumab, the one-armed humanized antibody against c-Met, blocks the interaction between the HGF-α chain and the Sema domain of the c-Met receptor. Clinical trials are also ongoing for two bivalent antibodies against c-Met (emibetuzumab and ARGX-111). More important aspects to consider for the development of c-Met therapeutics include the identification of effective combinations of inhibitors targeting the HGF/c-Met pathway along with other interacting signaling pathways such as the EGFR (epidermal growth factor receptor) pathway. ME22S, a bispecific antibody that targets EGFR and c-Met, was generated to induce an efficient EGFR internalization and degradation in the presence of Met that is frequently overexpressed in the invasive tumors and involved in the resistance against EGFR-targeted therapies.
Our Service
c-Met-based Bispecific ADCs Services
c-Met×EGFR bispecific ADCs have emerged recently, which seek to enhance internalization and trafficking to the lysosome, thus maximizing the amount of drug effectively delivered to tumor cells at a given dose. It has been demonstrated that selecting the appropriate combination of affinity optimized c-Met×tumor target bispecific ADC variants could lead to higher selectivity for tumor versus normal tissue, which could broaden the therapeutic index.
Creative Biolabs is a long-term expert who focuses on the discovery and development of novel ADCs. We have constructed different epitope combinations for c-Met-based bispecific ADCs development, including c-Met×EGFR, c-Met×HER2, c-Met×HER3, c-Met×PD-L1, and c-Met×VEGF. We are confident in providing the best service and the most suitable solution to satisfy your specific demands. If you are interested in our services, please do not hesitate to contact us for more details.
Highlights
- Proven Efficacy: Our bispecific ADCs have shown enhanced drug selectivity and efficacy in preclinical cancer studies, providing a promising therapeutic approach for cancer treatment.
- Comprehensive Expertise: With years of experience in ADC production, Creative Biolabs has successfully completed numerous ADC development projects, earning the trust of research institutions and large biotech companies globally.
- c-Met Focus: Our c-Met-based bispecific ADCs leverage the HGF/c-Met signaling pathway, targeting various cellular processes like proliferation and survival, making c-Met a key therapeutic target in cancer treatment.
- Advanced ADC Platforms: Creative Biolabs offers a full range of c-Met-based bispecific ADC development services, including innovative combinations such as c-Met×EGFR, c-Met×HER2, and c-Met×PD-L1, ensuring tailored solutions for diverse research needs.
- Cutting-Edge Therapeutics: With ongoing preclinical and clinical trials of antibodies targeting c-Met, Creative Biolabs remains at the forefront of developing next-generation cancer therapies.
- Client-Centric Solutions: We provide customized ADC construction services to meet specific project requirements, offering the best service and most suitable solutions to advance your therapeutic goals.
FAQ
-
Q: What are c-Met-based bispecific ADCs and how do they work in cancer therapy?
A: c-Met-based bispecific ADCs are designed to target the c-Met receptor and another tumor-specific antigen, enhancing the selectivity and efficacy of the cytotoxic drug delivery. This dual-targeting approach improves internalization and lysosomal trafficking, effectively delivering the drug to cancer cells and minimizing off-target effects.
-
Q: Why is c-Met an important target in cancer treatment?
A: c-Met, or hepatocyte growth factor receptor (HGFR), is involved in various cellular processes such as proliferation, differentiation, and survival. Overexpression of c-Met is associated with the development and progression of several cancers, making it a promising therapeutic target for antibody-drug conjugates (ADCs).
-
Q: How does Creative Biolabs enhance the development of c-Met-based bispecific ADCs?
A: Creative Biolabs leverages its extensive experience in antibody discovery and bioconjugation to develop high-affinity c-Met-based bispecific ADCs. By optimizing epitope combinations and utilizing advanced platforms, they ensure efficient internalization, lysosomal trafficking, and potent anti-tumor activity of the ADCs.
-
Q: What are some examples of c-Met-based bispecific ADCs currently in development?
A: Examples include c-Met×EGFR, c-Met×HER2, c-Met×HER3, c-Met×PD-L1, and c-Met×VEGF bispecific ADCs. These combinations are designed to enhance selectivity for tumor cells, improve internalization, and maximize drug delivery, thereby increasing therapeutic efficacy.
-
Q: What services does Creative Biolabs offer for c-Met-based bispecific ADCs development?
A: Creative Biolabs provides comprehensive services, including the selection of epitope combinations, antibody engineering, drug conjugation through their DrugLnk™ platform, and thorough in vitro and in vivo analysis. Their expertise ensures the development of highly effective c-Met-based bispecific ADCs tailored to specific project requirements.
-
Q: How does Creative Biolabs optimize the combination of epitopes for c-Met-based bispecific ADCs?
A: Creative Biolabs employs a strategic approach to selecting and optimizing epitope combinations for c-Met-based bispecific ADCs. By targeting c-Met in conjunction with other tumor-specific antigens like EGFR, HER2, HER3, PD-L1, and VEGF, they enhance the selectivity and internalization of the ADCs, ensuring effective delivery of the cytotoxic payload to cancer cells while minimizing impact on normal tissues.
Published Data
In the experiment, EGFR×c-MET bispecific antibody-drug conjugates (ADCs) were engineered to enhance tumor selectivity and efficacy by targeting both EGFR and c-MET. These bispecific ADCs were designed to improve therapeutic outcomes by simultaneously engaging two receptor tyrosine kinases that are overexpressed in various cancers and associated with poor prognosis. The study demonstrated that these bispecific ADCs exhibited high in vitro selectivity toward tumor cells expressing both antigens and displayed potent anti-tumor efficacy. By reducing the affinity for EGFR, the bispecific ADCs minimized toxicity in normal tissues, such as keratinocytes. This approach showed promise in enhancing the therapeutic index and reducing adverse effects, suggesting that EGFR×c-MET bispecific ADCs could provide a more effective and safer treatment option for cancers overexpressing these receptors.
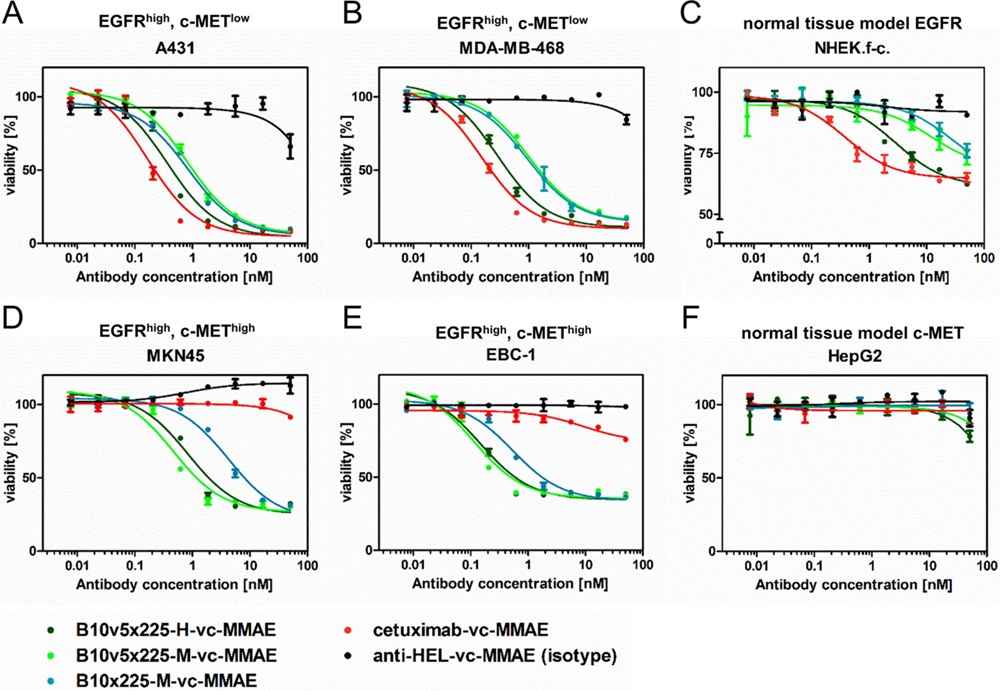 Fig.2 Cytotoxicity of EGFR×c-MET bispecific SEED antibody-drug conjugates.2
Fig.2 Cytotoxicity of EGFR×c-MET bispecific SEED antibody-drug conjugates.2
Featured Products
Anti-c-MET ADC
| Catalog | Product Name | Antibody |
| ADC-W-1538 | Anti-MET (Onartuzumab)-SMCC-DM1 ADC | Humanized Anti-MET IgG1 monovalent antibody, Onartuzumab |
| ADC-W-1539 | Anti-MET (Onartuzumab)-SPDB-DM4 ADC | Humanized Anti-MET IgG1 monovalent antibody, Onartuzumab |
| ADC-W-1540 | Anti-MET (Onartuzumab)-MC-MMAF ADC | Humanized Anti-MET IgG1 monovalent antibody, Onartuzumab |
| ADC-W-1541 | Anti-MET (Onartuzumab)-MC-Vc-PAB-MMAE ADC | Humanized Anti-MET IgG1 monovalent antibody, Onartuzumab |
| ADC-W-1542 | Anti-MET (Onartuzumab)-MC-Vc-PAB-SN38 ADC | Humanized Anti-MET IgG1 monovalent antibody, Onartuzumab |
References
- Zhang Y.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Molecular Cancer, 2018, 17(1):45.
- Sellmann, Carolin, et al. "Balancing selectivity and efficacy of bispecific epidermal growth factor receptor (EGFR)× c-MET antibodies and antibody-drug conjugates." Journal of Biological Chemistry 291.48 (2016): 25106-25119.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
