At Creative Biolabs, we specialize in providing virus-like particle (VLP) production services for viral infection research. VLPs, while mimicking the natural virus structure, are non-infectious, a unique characteristic that makes them a safe and efficient tool in research and vaccine development.
Virus-like particles (VLPs) are primarily composed of the structural proteins of the virus, which have the ability to self-assemble into VLP structures. VLPs can be a simple combination of a single protein type or may contain complex structures of multiple proteins. In their assembly, no viral genetic material is involved, meaning the resulting VLPs contain no RNA or DNA. This key characteristic implies that they are incapable of replication and do not cause viral infection.
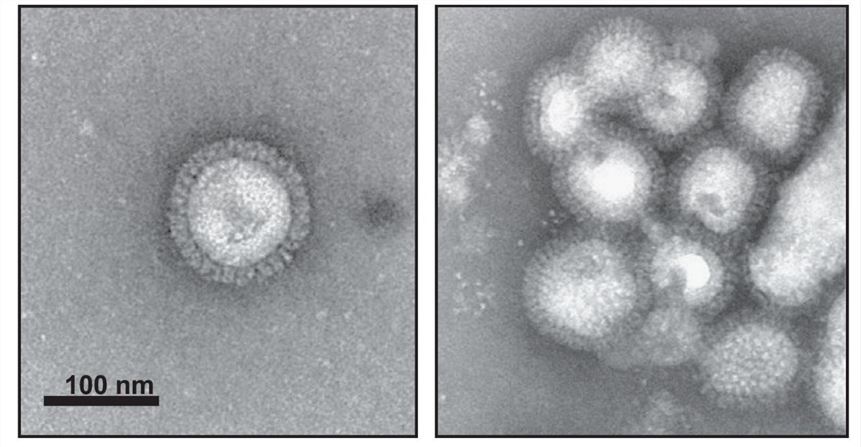 Fig.1 Electron microscopy images of influenza VLPs prepared in Sf9 cells using baculovirus expression system.1,3
Fig.1 Electron microscopy images of influenza VLPs prepared in Sf9 cells using baculovirus expression system.1,3
In our advanced laboratories, we offer efficient VLP preparation services based on the HEK293 expression system. Our service process is carefully designed to ensure the highest standards from gene optimization to the final VLP preparation.
1. Gene Optimization
We first optimize the expression genes of the viral structural proteins to ensure their optimal expression in HEK293 cells. This includes adjusting the gene sequence to enhance its transcription and translation efficiency, thereby enhancing the yield and quality of VLPs.
2. Vector Construction
3. Feasibility Experiment
| Particle size detection | Transmission electron microscopy observation | Bicinchoninic Acid assay |
| High-performance liquid chromatography testing | Western Blot | Enzyme-Linked Immunosorbent Assay |
| In vitro immunogenicity testing | …… |
5. Large-scale Preparation and Purification of VLPs
After successful preliminary experiments, we will undertake large-scale production of VLPs to meet clients' needs in research or clinical trials.
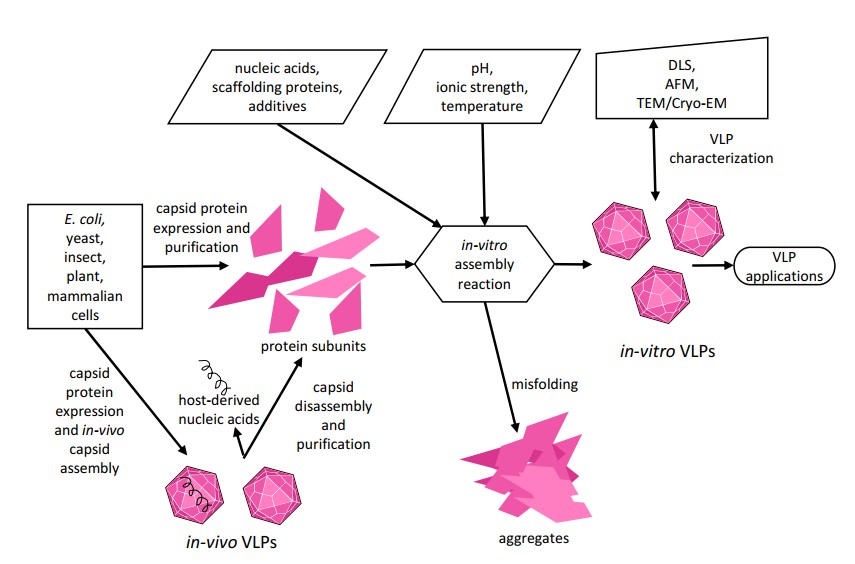 Fig.2 Schematic representation of in vitro VLP assembly.2,3
Fig.2 Schematic representation of in vitro VLP assembly.2,3
Creative Biolabs has been at the forefront of infectious disease research, and our VLP preparation services are notable for their excellent cost-effectiveness. Our unique preparation process not only ensures that VLPs maintain a stable natural conformation but also guarantees the high uniformity of their particle size, enhancing their reliability in research and application while maintaining biological activity. If you are looking for high-quality VLPs, feel free to contact us.
References: