Pseudovirus Production Service for Virus Infection Research
In the field of modern virology research, pseudovirus technology has become an important tool for virus infection research and antiviral drug screening due to its unique safety and efficiency. As a pioneer in the biotechnology field, Creative Biolabs is dedicated to providing comprehensive pseudovirus production services for researchers around the world, aiming to support the research of virus infection mechanisms and the development of vaccines and antibody drugs.
What are Pseudoviruses?
Pseudoviruses refer to virus-like particles that can mimic the process of real virus invading cells but lack the ability to replicate. They are usually constructed from modified viral genomes and can integrate key surface proteins of other viruses, such as HPV's L1 and L2 proteins, thereby used to study how viruses interact with host cells. The use of pseudoviruses reduces the requirements for biosafety levels and lowers the risk of experiments, making it a safe and effective research method.
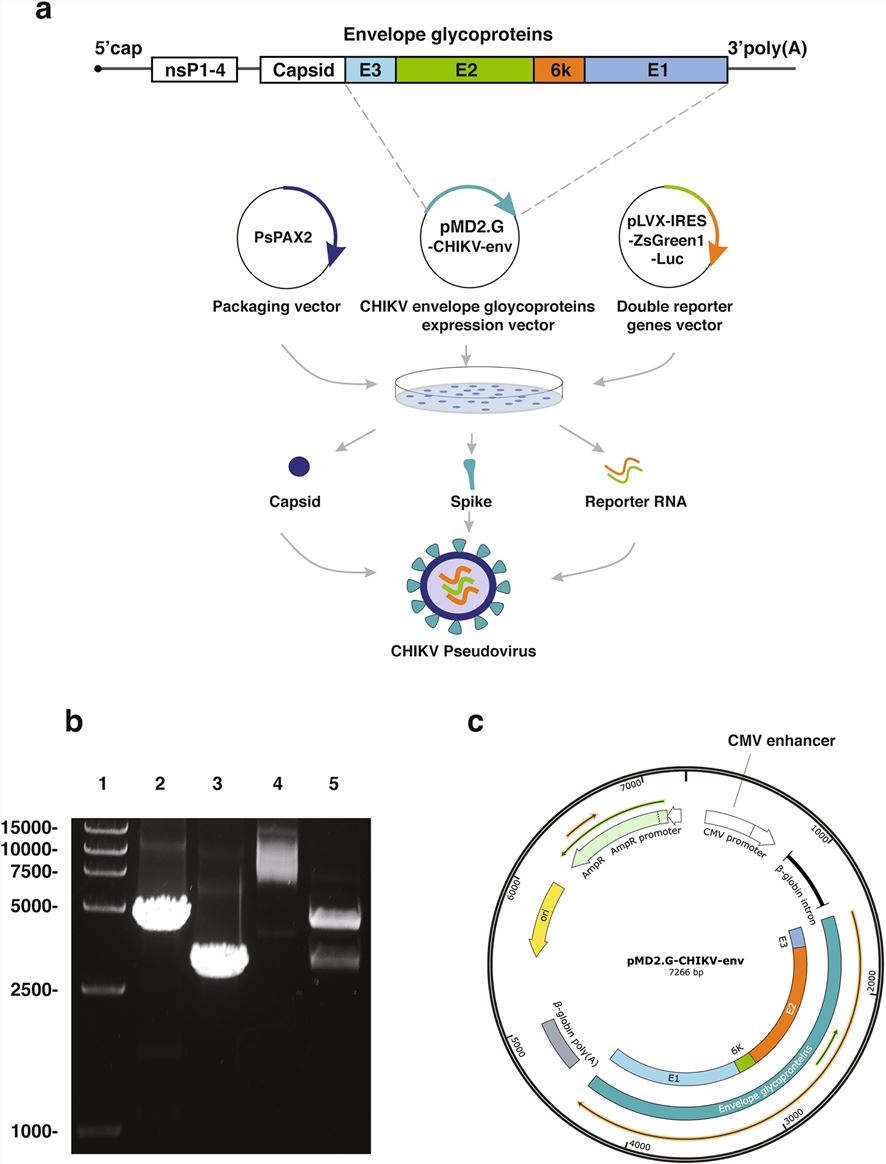 Fig.1 Construction of the chikungunya(CHIKV)pseudovirus.1,2
Fig.1 Construction of the chikungunya(CHIKV)pseudovirus.1,2
Pseudovirus Production Service Process at Creative Biolabs
-
Initial Consultation and Requirement Analysis: Our expert team communicates in-depth with clients to establish research objectives and requirements.
-
Pseudovirus Design and Construction: Design and construct pseudoviruses with specific surface proteins based on the characteristics of the virus and the needs of the client.
-
Pseudovirus Packaging and Purification: Use professional cell lines for pseudovirus packaging, and ensure their purification and stability through various methods.
-
Quality Control and Titer Determination: Strict quality control processes ensure the quality of pseudovirus products, and titer determination is conducted to meet experimental needs.
-
Product Delivery: Deliver pseudovirus products and corresponding quality inspection reports to clients within the agreed time.
Applications of Pseudoviruses
-
Setting the Lower Detection Limit: Improve the sensitivity and accuracy of experiments by precisely determining the lower detection limit of pseudoviruses.
-
Standardization of Nucleic Acid Testing: pseudoviruses serve as positive controls for nucleic acid testing, internal reference standards, or negative controls for assessing cross-reactions, ensuring the accuracy and reliability of detection.
-
Laboratory Testing Method Validation and Quality Control: Use pseudoviruses for the validation and evaluation of laboratory testing methods, as well as quality control processes, to standardize and regulate scientific research and clinical testing.
-
Neutralization Capacity Identification: Provide professional pseudovirus neutralization inhibition rate testing services to comprehensively evaluate the neutralization potential and efficacy of antibodies, peptide molecules, or sera.
-
Antivirus Efficacy Evaluation: Conduct comprehensive antiviral efficacy analysis of candidate drugs to predict their clinical application prospects.
-
Virus-Host Cell Receptor Interaction Research: Explore in depth the binding mechanism between pseudoviruses and host cell receptors, providing key information for the analysis of virus invasion mechanisms.
-
Antibody Screening and Functional Evaluation: Use pseudovirus tools to screen potential antibodies and comprehensively evaluate their functions.
-
Vaccine Efficacy Evaluation: Study the quality of immune responses elicited by vaccines, and assess the neutralization capacity of the induced antibodies against various virus variants, providing scientific evidence for vaccine development.
Service Advantages
-
Technically Mature, Rich Experience: Many years of experience in pseudovirus research and service, providing professional services to numerous scientific institutions and pharmaceutical companies worldwide.
-
A Large Stock of Pseudoviruses: Our pseudovirus product line is extensive, catering to immediate needs and ensuring no delay in research.
-
Customized Solutions: Provide customized experimental design and solutions based on the specific research needs of clients.
-
High Safety: All pseudovirus products are produced in Biosafety Level 2 laboratories, ensuring experimental safety.
-
Short Service Cycle, High Throughput: Efficient service processes and high-throughput detection capabilities guarantee the completion of a large number of sample tests in a short time.
-
Customer Support and Service: Provide full-range technical support and high-quality customer service.
Creative Biolabs is always committed to providing high-quality pseudovirus production services comparable to real viruses for virology research and antiviral drug development. If you need customized pseudoviruses or want to learn more about related information, please feel free to contact us.
References:
-
Su, C.; et al. Preparation and application of chikungunya pseudovirus containing double reporter genes. Scientific Reports. 2022, 12(1):9844.
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only. We do not
provide direct services or products for patients.
Related
Services:
 Fig.1 Construction of the chikungunya(CHIKV)pseudovirus.1,2
Fig.1 Construction of the chikungunya(CHIKV)pseudovirus.1,2