In Vitro Micronucleus Assay Service
Micronucleus Assay for Evaluation of Genetic Toxicology
The micronucleus assay is a commonly utilized technique for assessing genotoxicity, particularly in identifying DNA damage and chromosomal abnormalities within cells. The presence of micronuclei in a cell indicates DNA damage and genotoxic effects, as they are small additional nuclei that form outside of the main nucleus during cell division. These micronuclei contain fragments of chromosomes that are not incorporated into the main nucleus. A micronucleus assay is a valuable tool in assessing genotoxicity and evaluating the potentially harmful effects of various compounds on the DNA and chromosomal integrity of cells. It is often used as an alternative or complementary method to the in vitro chromosome aberration assay.
Micronucleus Assay Service
Creative Biolabs provides in vitro micronucleus assay services for genotoxicity testing. The micronucleus assay is a well-established technique used in assessing DNA damage and chromosomal abnormalities in cells. Our assays are performed using Chinese hamster ovary-K1 (CHO-K1) cells. The cells are stained and then imaged using a high-content analysis system, which allows for the automated acquisition of images and analysis of multiple parameters such as number of micronuclei per cell, cell morphology, and nuclear size. This high-throughput approach enables the screening of genotoxic compounds and metabolites and the evaluation of their impact on chromosomal integrity.
Some chemicals may be directly genotoxic, while others require metabolic activation to become genotoxic. When assessing the genotoxicity of metabolites using the micronucleus assay, it is important to consider the metabolic activation of these metabolites. The S9 fraction is a mix of enzymes derived from the liver that are typically used in in vitro genotoxicity assays to mimic the metabolic activation that occurs in vivo. By utilizing the S9 fraction in the micronucleus assay, researchers can better assess the genotoxic potential of metabolites that require metabolic activation to induce DNA damage.
Summary of Our Test Method
The test is typically performed using cultured mammalian cells such as Chinese hamster ovary (CHO) cells or human lymphocytes. Treat cells with appropriate concentrations of the test substance in the presence/absence of the S9 metabolic activation system for a short period, typically 3-6 hours. Following treatment, wash the cells to remove the test substance and allow them to recover in fresh media for an appropriate post-treatment period. Finally, the cells are fixed and stained to visualize the chromosome structure. Micronuclei, which are small extra nuclei that form in the cytoplasm of cells, are then counted and scored as a marker of genetic damage. The frequency of micronuclei is compared to controls to determine the genotoxicity of the test substance.
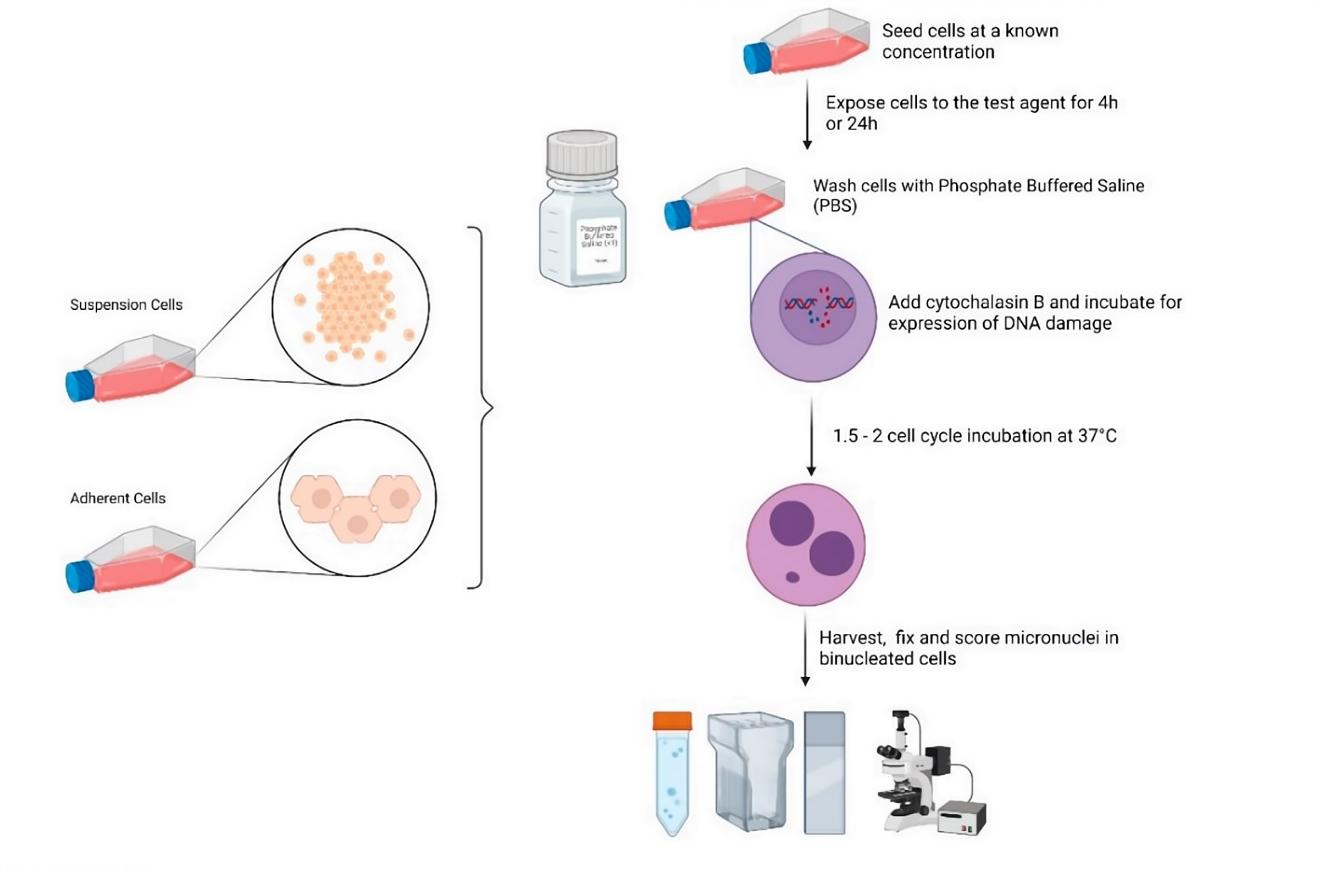 Fig.1 The standard workflow of micronucleus assay.1
Fig.1 The standard workflow of micronucleus assay.1
Creative Biolabs offers a wide range of in vitro ADME assays to support drug development and safety evaluation. Whether you require in vitro ADME screening, assessing drug metabolic stability and drug distribution, or evaluating drug-drug interactions, our skilled team provides professional testing and the critical data you need. Contact us today to learn more about how we can assist in advancing your drug development efforts.
Reference
-
Farabaugh, Christopher S., et al. "In vitro micronucleus assay: method for assessment of nanomaterials using cytochalasin B." Frontiers in Toxicology 5 (2023): 1171960.
For Research Use Only | Not For Clinical Use


 Fig.1 The standard workflow of micronucleus assay.1
Fig.1 The standard workflow of micronucleus assay.1
 Download our brochure
Download our brochure