Non-rodent In Vivo PK Service
Non-rodent In Vivo PK
Non-rodent in vivo pharmacokinetic (PK) studies involve assessing the behavior of drugs within living organisms that are not rodents, such as dogs, non-human primates (NHPs), pigs, rabbits, or other species. These studies are essential in preclinical drug development to understand how drugs are absorbed, distributed, metabolized, and excreted in non-rodent species, which can provide valuable insights into human pharmacokinetics. Non-rodent in vivo PK studies provide valuable insights into drug behavior, efficacy, and safety across various therapeutic areas, complementing rodent models and enhancing the translational relevance of preclinical research.
Our Service
Creative Biolabs provides expertise, state-of-the-art instrumentation facilities, and abundant resources for preclinical PK studies in non-rodent species for drug development and research purposes. Here are some key services of non-rodent in vivo PK studies:
Consultation and Study Design
-
Creative Biolabs designs custom PK studies based on the client's specific research goals and requirements. This includes the selection of appropriate non-rodent species, study design, dosing regimen, and sampling schedule.
Animal Procurement
-
Creative Biolabs offers different non-rodent species, ensuring the welfare and well-being of the experimental animals throughout the study period.
Dosing and Drug Administration
-
Creative Biolabs assists with drug formulation, dose selection, and route of administration to ensure accurate and reproducible drug delivery to non-rodent species.
Sample Collection and Analysis
-
Creative Biolabs collects blood samples as well as other biological specimens. State-of-the-art analytical techniques, such as liquid chromatography-mass spectrometry (LC-MS), high-performance liquid chromatography (HPLC), or enzyme-linked immunosorbent assay (ELISA), are used to quantify drug concentrations in these samples.
Pharmacokinetic Analysis
-
Creative Biolabs analyzes the collected data to calculate key pharmacokinetic parameters, including Cmax, Tmax, AUC, t1/2, CL, and Vd, to characterize the pharmacokinetic profile of the drug in non-rodent species.
Data Interpretation and Reporting
-
Creative Biolabs delivers a comprehensive report summarizing the study methodology, results and conclusions and providing recommendations for further research or development.
Project Management and Communication
-
Creative Biolabs' dedicated project managers facilitate communication between clients and researchers, provide updates, resolve issues, and ensure projects are completed in a timely manner.
All studies are conducted in compliance with relevant regulatory guidelines, such as Good Laboratory Practice (GLP) or other applicable standards, to ensure the reliability, integrity, and regulatory acceptance of study data.
Species for Non-rodent Pharmacokinetics
Non-rodent species have unique advantages and physiological characteristics that in some cases can better mimic human pharmacokinetics. Here are some common non-rodent species used in pharmacokinetic studies:
 Fig.2 Species for non-rodent pharmacokinetics.
Fig.2 Species for non-rodent pharmacokinetics.
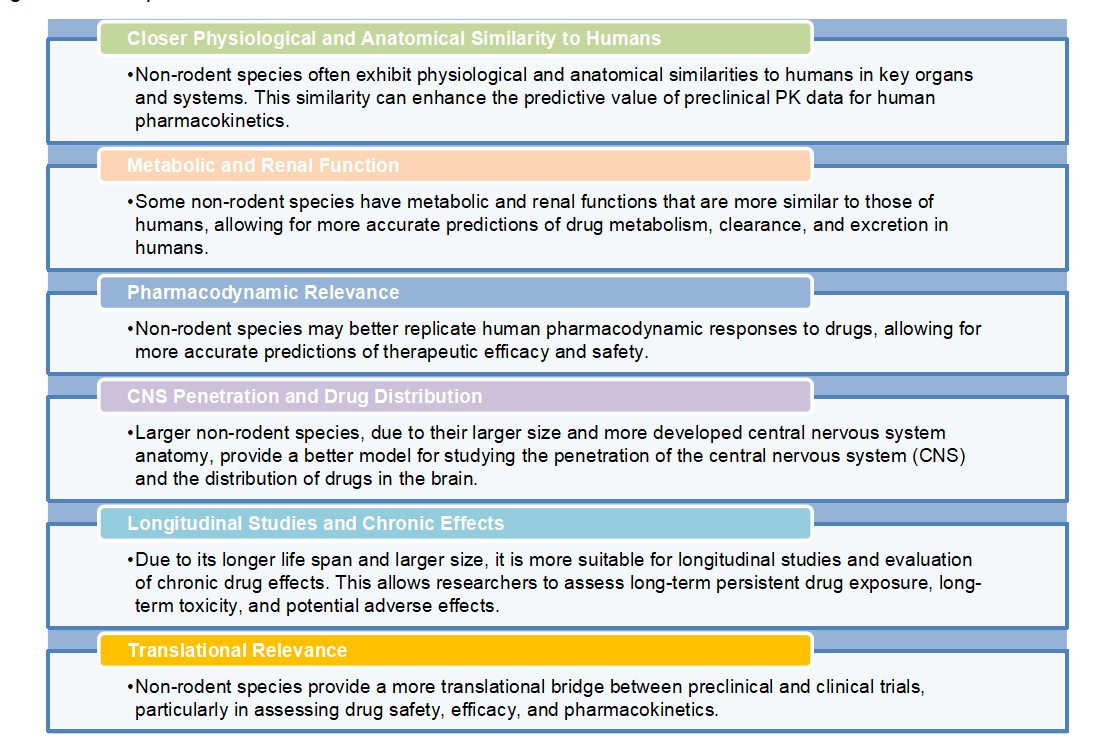
Advantages of Non-rodent Species
The advantage of nonrodents stems from their diverse physiology, anatomy, and genetic makeup, which allows for better mimicking human pharmacokinetics and provides additional insights into drug behavior. Here are some of the advantages of using non-rodent species in PK studies:
Why Choose Creative Biolabs
 Fig.3 Our advantages.
Fig.3 Our advantages.
Frequently Asked Question
Q1: How to choose non-rodent species?
A1: The choice of non-rodent species for pharmacokinetic studies depends on various factors, including the specific research objectives, drug properties, anatomical and physiological similarities to humans, ethical considerations, availability, and practical constraints. Each species offers unique advantages and may be best suited for specific research questions or experimental conditions.
Click here to learn more about rodent in vivo PK service! If you still have questions about your project, you can contact our animal research scientists today for assistance.
For Research Use Only | Not For Clinical Use



 Fig.2 Species for non-rodent pharmacokinetics.
Fig.2 Species for non-rodent pharmacokinetics.

 Fig.3 Our advantages.
Fig.3 Our advantages.
 Download our brochure
Download our brochure