Rhesus Monkey Serum (NHP-BP002)

Tag: Rhesus Monkey, Serum
- Rhesus monkey is often used in biological and medical research related to human and animal diseases even space travel due to its high closeness to humans. This monkey-derived product is collected from Rhesus monkeys. The sample is confirmed as negative for Herpes B Virus, retroviruses, SIV, SRV, and STLV. It can be used as a tool in a life science laboratory to conduct biochemical experiments such as blocking, ELISA, ICC/IF, IHC, and WB.
Detailed Product Description
- Source: Healthy rhesus monkey
- Purity: No hemolysis or clotting.
- Applications: Suitable for various research fields including biomarker research, pharmacology, and toxicology.
- Storage Conditions: Store at -20 °C or -80 °C. Avoid freeze-thaw cycle.
Technical Specifications
- Collection Method: Serum is collected from rigorously screened healthy donors, and the collection process strictly adheres to animal welfare requirements. Obtained through centrifugation processing of non-anticoagulated whole blood, serum does not contain fibrinogen or other clotting factors and serves as a primary source for biomarkers.
- Quality Control: Strict accordance with biomedical standards.
- Available Formats: Frozen
The paper details a comprehensive study on the impact of treatment for iron deficiency anemia (IDA) with iron dextran injections in infant rhesus monkeys and its effects on the serum metabolomic profile. The study comprised a cohort of iron-sufficient (IS) and IDA rhesus infants, which looked at changes from their metabolomic profiles post-treatment with iron dextran combined with vitamin B injections.
Among the key experimental findings were: This means that, prior to treatment, infants with IDA had a disordered serum metabolomic profile with altered liver metabolism, bile acid release, and nucleoside levels. Post-treatment evaluations showed normalization of traditional indices like hemoglobin and mean corpuscular volume, which confirmed the effectiveness of iron dextran in correcting hematologic aspects of IDA.
However, after correcting for the same, the metabolomic profile of the treated IDA group continued to exhibit substantial change, with 323 out of 654 metabolites showing altered expression relative to baseline. These changes were predominantly seen across several biochemical pathways, implicating a profound metabolic reprogramming beyond hemoglobin normalization. The research underlines that the treatment of iron-deficient young subjects with iron preparations is far from being a trivial issue. It points to the fact that, since iron preparations are capable of normalizing hematological deficiencies, they also induce a series of quite significant changes in metabolites, serum function of the liver, production of essential fatty acids, and neurological metabolites. Caution is exercised with this study, which points out the need for cautious consideration of treatment regimens for IDA, especially in infants. This comes from the fact that its rapid alteration of iron rapidly affects broad systemic impacts.
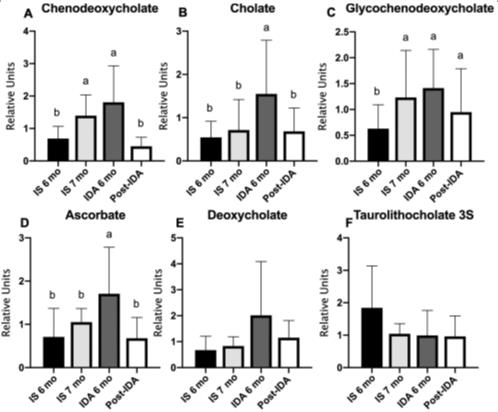 1Fig. 1 Bile acid and other liver metabolites were altered by iron deficiency anemia and iron treatment.
1Fig. 1 Bile acid and other liver metabolites were altered by iron deficiency anemia and iron treatment.
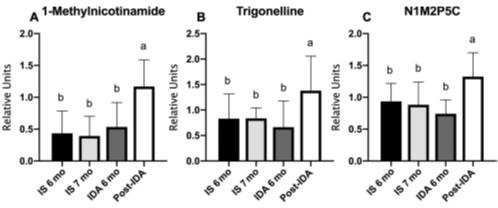 1Fig. 2 NAD metabolites in IDA infants are increased after iron administration.
1Fig. 2 NAD metabolites in IDA infants are increased after iron administration.
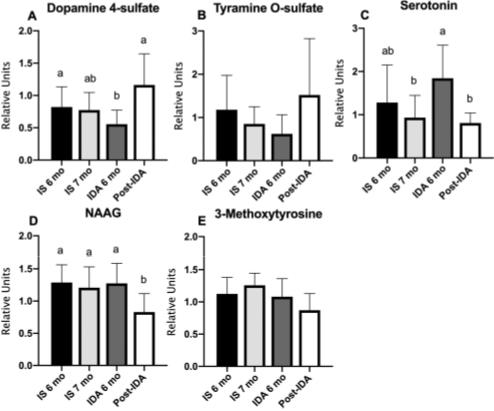 1Fig. 3 Neuroactive metabolites are altered with IDA and treatment. Metabolomic determinations of (A) dopamine 4-sulfate, (B) tyramine O-sulfate, (C) serotonin, (D) NAAG, and (E) 3-methoxytyrosine in IS or IDA infants before and after treatment.
1Fig. 3 Neuroactive metabolites are altered with IDA and treatment. Metabolomic determinations of (A) dopamine 4-sulfate, (B) tyramine O-sulfate, (C) serotonin, (D) NAAG, and (E) 3-methoxytyrosine in IS or IDA infants before and after treatment.
Reference
- Reference: Brian J Sandri., et al. " Correcting iron deficiency anemia with iron dextran alters the serum metabolomic profile of the infant Rhesus Monkey." The American Journal of Clinical Nutrition (2021) 915 –923
-
Q 1: What are the key considerations for using Rhesus Monkey Serum in research?
A: Researchers need to consider ethical sourcing, compatibility with their specific study goals, and ensuring minimal variation between batches to maintain experiment consistency.
-
Remarkable Quality and Consistency
We've been using Rhesus Monkey Serum for comparative immunological studies. The quality and consistency have been remarkable, aiding significantly in our research accuracy. -
Excellent Support.
Rhesus Monkey Serum from Creative Biolabs has proven to be invaluable for our vaccine development projects. The excellent support and product reliability have made a huge difference in our work.
Click the button below to contact us or submit your feedback about this product.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Enter your email here to subscribe.
SubmitFollow us on


 Cynomolgus Monkey PBMCs
Cynomolgus Monkey PBMCs