A comprehensive protein engineering platform employs advanced computational and experimental techniques to design, optimize, and produce proteins with desired properties for various applications in medicine, industry, and research. It integrates molecular modeling, gene synthesis, expression systems, purification, high-throughput screening, display technologies for screening and evolution, Computer-Aided Drug Design (CADD) for rational design, and structural analysis techniques like protein crystallization. This multidisciplinary approach accelerates the development of novel proteins with tailored functions, driving advancements in biotechnology and medicine.
Display technology for screening and evolution is a powerful set of techniques used in protein engineering to identify and evolve proteins with desirable traits. These technologies enable the rapid screening of vast libraries of protein variants, facilitating the selection of proteins with optimal characteristics. Cell surface display technology is a key method in this field, which immobilizes functional proteins or peptides on microbial cells by fusing them with anchoring genes and expressing them on the cell surface or plasma membrane using signal peptides. As a cornerstone of protein engineering, display technology accelerates the screening and evolution of proteins with desired traits. Various display technologies are utilized, each offering unique advantages.
Phage Display
Phage display is a technique that presents peptides or proteins on the surface of bacteriophages. In this technique, libraries of protein variants are fused to a coat protein of the phage, enabling the display of these variants on the phage surface. This method is widely used for antibody engineering, identifying protein-protein interactions, and evolving proteins with improved binding affinity or specificity. Its advantages include the ability to handle large library sizes, an efficient selection process, and compatibility with a diverse range of proteins.
Yeast Surface Display
In yeast display, protein variants are expressed on the surface of yeast cells, with the protein of interest fused to an agglutinin protein that anchors it to the yeast cell wall. This method is used for engineering antibodies, enzymes, and other proteins, and is also valuable for studying protein interactions and stability. Its advantages include the ability to achieve proper protein folding and post-translational modifications due to the eukaryotic expression system.
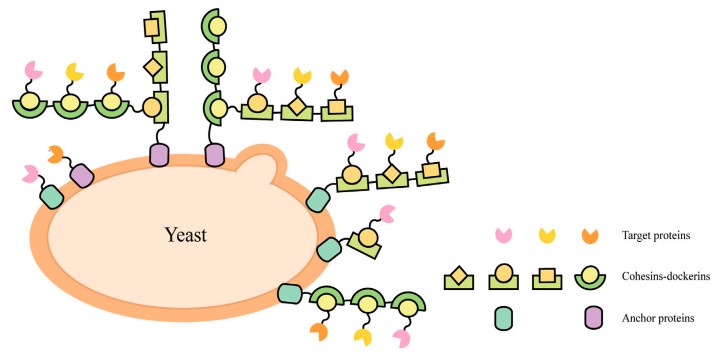 Fig.1 Schematic illustrations of Yeast-surface display.1,3
Fig.1 Schematic illustrations of Yeast-surface display.1,3
Bacterial Display
Bacterial display involves expressing proteins on the surface of bacterial cells, typically E. coli, similar to yeast display. This method is used for screening large libraries of peptides and proteins, particularly in binding assays and enzyme engineering. Its advantages include the rapid growth and ease of manipulation of bacterial cells, as well as the capability to handle large library sizes.
Mammalian Display
Mammalian cell display involves presenting proteins on the surface of mammalian cells, providing a more relevant context for human proteins, including proper folding and post-translational modifications. This method is particularly useful for engineering therapeutic proteins and antibodies. Its main advantage is that it offers a biologically relevant environment for screening proteins intended for use in humans.
CADD is critical in modern drug discovery, leveraging computational tools and techniques to discover, design, and optimize new therapeutic compounds. By combining computational power with experimental data, CADD continues to revolutionize the field of drug discovery, offering new hope for developing effective and safe therapeutic agents. The main objective of CADD is to accelerate drug discovery, lower costs, and improve the likelihood of identifying effective drug candidates.
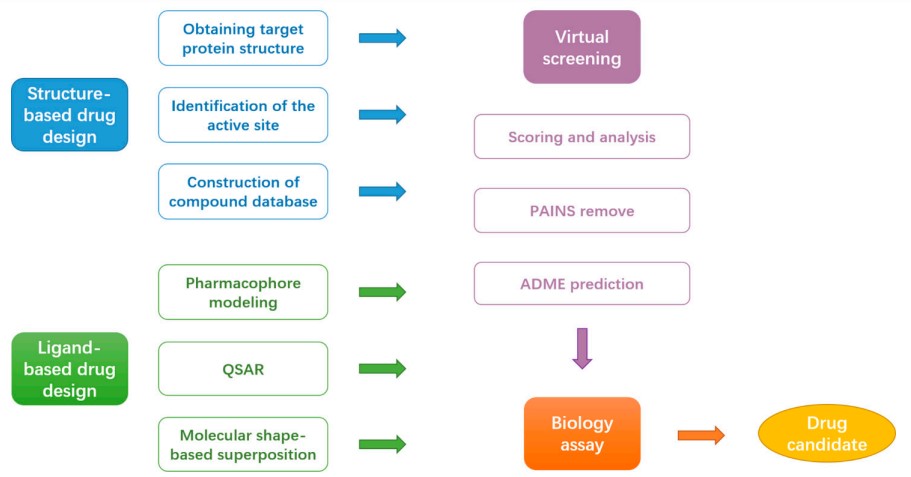 Fig.2 The Flow chart of commonly used CADD approaches.2,3
Fig.2 The Flow chart of commonly used CADD approaches.2,3
CADD encompasses a variety of computational approaches that can be broadly classified into two main categories:
SBDD methods leverage the 3D structural information of macromolecular targets, such as proteins or RNA, to pinpoint critical sites and interactions crucial for their biological functions. This structural insight is then used to design antibiotic drugs that can interfere with these essential interactions, disrupting the biological processes vital for microorganism survival. SBDD focuses on understanding the 3D structure of these targets, primarily proteins, and employs various key techniques to achieve this goal.
LBDD methods center on analyzing known antibiotic ligands to determine the relationship between their physicochemical properties and their biological activities, known as structure-activity relationships (SAR). This information aids in optimizing current drugs and guides the development of new drugs with improved efficacy. LBDD is used when the structure of the target is not known but information about other molecules that bind to the target is available. Techniques include:
Owing to this powerful Protein Engineering Platform, Creative Biolabs provides additional aspects of protein engineering, including efficient expression systems such as bacterial, yeast, insect, and mammalian cells, to produce sufficient quantities of engineered proteins for diverse applications. We also offer in vitro and in vivo functional assays to validate the activity, stability, and specificity of these proteins, ensuring to meet the desired criteria for practical applications. Just feel free to contact us at any time at your convenience.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.