- Home
- Services
- Bispecific ADCs Development
- Biparatopic Receptor-based Bispecific ADCs
- Development of Biparatopic HER2-targeted Bispecific ADCs
Development of Biparatopic HER2-targeted Bispecific ADCs
Antibody-drug conjugates (ADCs) can deliver cytotoxic drugs specifically into targeted cells through internalization and lysosomal trafficking, which has been regarded as an effective cancer therapy strategy. With years of experience in the field of antibody discovery and bioconjugation, Creative Biolabs represents one of the leaders in the field of small molecular synthesis, antibody engineering as well as ADC development. Currently, we provide a novel ADC development service, which is named biparatopic bispecific ADCs that target two different non-overlapping epitopes of HER2. We believe our services will effectively save the time and cost of your programs by taking advantage of our expertise and innovative technology platforms.
Background
Introduction of HER2
HER2 (also known as receptor tyrosine-protein kinase erbB-2, CD340, proto-oncogene Neu or ERBB2), is a protein encoded by the ERBB2 gene located on the long arm of chromosome 17. HER2 is a member of the human epidermal growth factor receptor (EGFR) family that consists of three other receptors, epidermal growth factor receptor (HER1, erbB1), HER2 (erbB2), HER3 (erbB3), and HER4 (erbB4). HER2 is aberrantly overexpressed in certain malignancies such as breast and gastric cancer. It is overexpressed in around 20-30% of breast cancer tumors. HER2 is considered as an oncogenic driver in HER2 amplified cancers since it involves promoting tumorigenesis through multiple mechanisms. Dysregulated HER2 expression often results in an increased risk of recurrence and poorer prognosis in cancers. In HER2-positive malignancies, it can stimulate downstream RAS-RAF-ERK and PI3K-PTEN-AKT signaling to facilitate cancer cell proliferation. Thus, HER2 has become an important biomarker and therapeutic target in cancer diagnosis and targeted treatment such as the ADCs.
 Fig.1 Extracellular Domains of HER2 and Antibody Binding.1
Fig.1 Extracellular Domains of HER2 and Antibody Binding.1
Biparatopic HER2-targeted Bispecific ADC
Following ado-trastuzumab emtansine (T-DM1), multiple novel HER2-targeted ADCs have been developed and shown the promise of improved potency and efficacy. At present, biparatopic HER2-targeted bispecific ADCs represent a new format in these ADCs. For example, a bivalent biparatopic antibody targeting two non-overlapping epitopes on HER2 conjugated to a tubulysin-based microtubule inhibitor can induce significant HER2 receptor clustering and enhance robust internalization, lysosomal trafficking, and degradation. This biparatopic ADC demonstrates potent anti-tumor activity over T-DM1 in tumor models. Therefore, the biparatopic ADC is considered as a promising potential therapy for metastatic breast cancer. Besides, safety studies in non-human primates demonstrated that the biparatopic ADC is sufficient to enter clinical trials. To date, a phase I clinical trial to assess the safety and preliminary efficacy of the biparatopic ADC is underway in patients refractory to or ineligible for current HER2-targeted therapies.
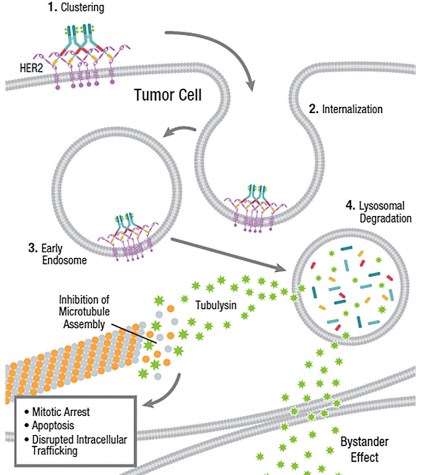 Fig.2 Mechanism of action (MOA) of biparatopic HER2-targeted bispecific ADC.2
Fig.2 Mechanism of action (MOA) of biparatopic HER2-targeted bispecific ADC.2
ZW49 is another anti-HER2 biparatopic ADC composed of an Azymetric anti-HER2 IgG1 linked to ZymeLink Auristatin via a protease cleavable linker. Preclinical study showed that ZW49 demonstrates significant lysosomal trafficking and superior internalization relative to the HER2-targeted monospecific ADC. Besides, this biparatopic ADC presents greater tolerability and exposure. These properties enable ZW49 to generate complete responses in HER2 low to high-expressing PDX models at exposures tolerated in non-human primates. So far, ZW49 has been evaluated in Phase 1 clinical trial since initiated in 2019.
Our Service
Biparatopic HER2-targeted Bispecific ADCs Services
ADCs presently are developed rapidly due to the powerful platform and optimized conjugation strategies, and ADCs are showing more and more promising therapeutic outcome. Creative Biolabs is committed to ADCs design and development for more than ten years. Now we provide the best biparatopic ADC construction services against HER2 with first-class antibody-drug conjugate platforms, and we promise to apply our first-hand expertise to your distinct project requirements. Our comprehensive ADCs development services include ADC Antibody Screening, DrugLnk™ Custom Synthesis, Antibody Design and Conjugation, ADC in vitro Analysis, and ADC in vivo Analysis. If you are interested in our services, please do not hesitate to contact us for more details.
Highlights
- Innovative Targeting Strategy: Our biparatopic HER2-targeted bispecific ADCs uniquely target two non-overlapping epitopes on HER2, enhancing receptor clustering, internalization, and lysosomal trafficking for superior therapeutic outcomes.
- Experienced Leadership: With over a decade of expertise in antibody discovery and bioconjugation, Creative Biolabs stands at the forefront of ADC development, ensuring high-quality and effective ADC solutions.
- Advanced Technology Platforms: Leveraging state-of-the-art technology platforms, our services offer optimized conjugation strategies, delivering more potent and efficient ADCs tailored to specific research and clinical needs.
- Comprehensive Development Services: Our extensive suite of services includes ADC Antibody Screening, DrugLnk™ Custom Synthesis, Antibody Design and Conjugation, and both in vitro and in vivo ADC analysis, providing a complete solution from design
- Cost and Time Efficiency: By utilizing our specialized expertise and innovative technology, we significantly reduce the time and cost of ADC development programs, accelerating the journey from research to clinical application.
FAQ
-
Q: Why is HER2 an important target in cancer therapy?
A: HER2, a member of the human epidermal growth factor receptor (EGFR) family, is overexpressed in several malignancies, including breast and gastric cancers. It promotes tumorigenesis through various signaling pathways, making it a crucial biomarker and therapeutic target in cancer diagnosis and treatment, particularly for developing antibody-drug conjugates (ADCs).
-
Q: How does Creative Biolabs enhance the development of biparatopic HER2-targeted bispecific ADCs?
A: Creative Biolabs utilizes its extensive experience in antibody discovery, engineering, and bioconjugation to develop high-affinity biparatopic HER2-targeted bispecific ADCs. Their advanced platforms and optimized conjugation strategies ensure efficient internalization, lysosomal trafficking, and potent anti-tumor activity of the ADCs.
-
Q: What are the advantages of biparatopic HER2-targeted bispecific ADCs over traditional ADCs?
A: Biparatopic HER2-targeted bispecific ADCs offer improved potency and efficacy by targeting two distinct epitopes on HER2, enhancing receptor clustering, internalization, and lysosomal trafficking. This results in more efficient drug delivery to cancer cells and greater anti-tumor activity compared to traditional ADCs.
-
Q: What services does Creative Biolabs offer for biparatopic HER2-targeted bispecific ADCs development?
A: Creative Biolabs provides comprehensive services, including ADC antibody screening, DrugLnk™ custom synthesis, antibody design and conjugation, in vitro and in vivo ADC analysis. Their expertise and advanced platforms ensure the efficient development of highly effective biparatopic HER2-targeted bispecific ADCs.
-
Q: How does Creative Biolabs ensure the safety and efficacy of biparatopic HER2-targeted bispecific ADCs?
A: Creative Biolabs conducts rigorous in vitro and in vivo analyses to evaluate the safety, internalization, lysosomal trafficking, and anti-tumor activity of biparatopic HER2-targeted bispecific ADCs. These evaluations ensure that the ADCs are both safe and effective for potential clinical applications.
Published Data
The study on Biparatopic anti-HER2 antibody-drug conjugates (ADCs) evaluated the potential therapeutic benefits of targeting two different epitopes of the HER2 receptor in breast cancer cells. The biparatopic ADCs were engineered to enhance receptor internalization, lysosomal trafficking, and degradation, thus improving cytotoxic payload delivery. In the experiments, the biparatopic ADCs showed superior anti-tumor activity compared to single epitope-targeting ADCs such as T-DM1, particularly in models where T-DM1 was ineffective. The combination of two domain-specific ADCs, pertuzumab-PEG6-DM1 and trastuzumab-PEG6-DM1, resulted in higher internalization and cytotoxicity, demonstrating greater potency against HER2-positive breast cancer cell lines, including those resistant to T-DM1. The study concluded that biparatopic targeting could be a promising strategy to overcome resistance and improve the efficacy of HER2-targeted therapies.
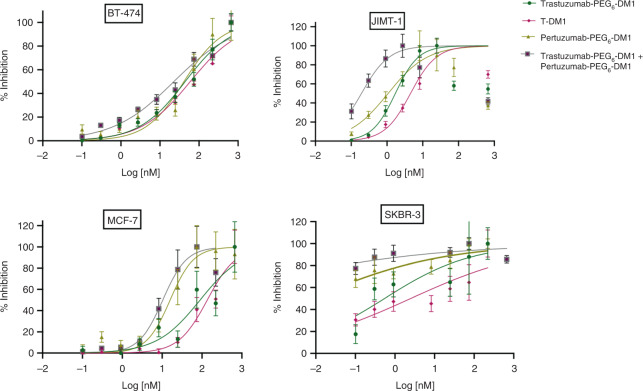 Fig.3 In vitro cytotoxicity of immunoconjugates in cells with different HER2 densities.3
Fig.3 In vitro cytotoxicity of immunoconjugates in cells with different HER2 densities.3
Featured Products
Anti-HER2 ADC
| Catalog | Product Name | Antibody |
| ADC-W-101 | Anti-ERBB2-Phor18 molecules ADC | lgG1 Anti-ERBB2 lgG1 antibody |
| ADC-W-116 | Anti-ERBB2 (Trastuzumab)-Gly5-modified DM1 ADC | Humanized Anti-ERBB2 lgG1 antibody, Trastuzumab |
| ADC-W-117 | Anti-ERBB2 (Trastuzumab)-vc-PAB-MMAE ADC | Humanized Anti-ERBB2 lgG1 antibody, Trastuzumab |
| ADC-W-149 | Anti-ERBB2 (clone hu4D5Fabv8)-Mc-VC-PABC-MMAF ADC | Anti-ERBB2 antibody, clone # hu4D5Fabv8 |
| ADC-W-150 | Anti-ERBB2 (clone hu4D5Fabv8)-Mc-MMAF ADC | Anti-ERBB2 antibody, clone # hu4D5Fabv8 |
References
- Damelin, Marc. "Introduction: Motivations for Next-Generation ADCs." Innovations for Next-Generation Antibody-Drug Conjugates (2018): 1-10.
- J. Y.; et al. A biparatopic HER2-targeting antibody-drug conjugate induces tumor regression in primary models refractory to or ineligible for HER2-targeted therapy. Cancer cell. 2016, 29(1): 117-129.
- Ketchemen, Jessica Pougoue, et al. "Biparatopic anti-HER2 drug radioconjugates as breast cancer theranostics." British Journal of Cancer 129.1 (2023): 153-162.
For Research Use Only. NOT FOR CLINICAL USE.

Online Inquiry
Welcome! For price inquiries, please feel free to contact us through the form on the left side. We will get back to you as soon as possible.
