Gene Therapy for Cardiovascular Disorders
Inquiry NowCardiovascular disorders, encompassing diseases that affect the heart and blood vessels, stand as the primary cause of death and disability globally. According to the World Health Organization, these disorders were responsible for an estimated 17.9 million deaths in 2019, constituting 32% of all global fatalities. Common conditions within this category include coronary artery disease, heart failure, and vein graft stenosis, characterized by impaired blood flow, reduced heart function, and an elevated risk of thrombosis, respectively. Although existing treatments, such as pharmacological therapies, surgical interventions, and mechanical devices, can mitigate symptoms and enhance quality of life, they fall short in addressing the root causes or preventing disease progression. Consequently, there is an urgent demand for the development of novel, effective therapies targeting the molecular and cellular mechanisms of cardiovascular disorders, aiming to restore the heart and blood vessels' normal functionality. Gene therapy emerges as a promising approach, involving the delivery of genetic material into cells to modify their gene expression or function. This method enables the correction or compensation of genetic defects and environmental factors contributing to cardiovascular disorders. Not only does gene therapy hold the potential to revolutionize treatment strategies, but it also offers hope in the quest for lasting solutions to these pervasive ailments.
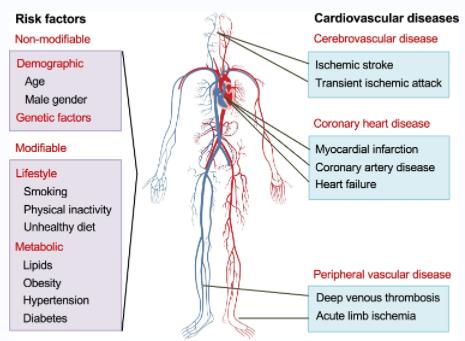 Fig.1 Cardiovascular Diseases and Their Risk Factors (Tabassum R, 2021)
Fig.1 Cardiovascular Diseases and Their Risk Factors (Tabassum R, 2021)
Gene Therapy for Coronary Artery Disease
Coronary artery disease (CAD) stands as the most prevalent cardiovascular disorder, caused by the accumulation of fatty plaques within the inner walls of coronary arteries, responsible for supplying blood and oxygen to the heart muscle. These plaques can narrow or block the arteries, leading to diminished blood flow and oxygen delivery, impairing heart function and triggering symptoms such as chest pain, shortness of breath, and heart attacks. Current CAD treatments encompass medications like anti-platelet drugs, statins, and nitrates, aimed at preventing blood clots, reducing cholesterol levels, and dilating arteries. However, these medications do not reverse plaque buildup or restore normal coronary artery function. Surgical interventions, such as coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), provide options to bypass or open blocked arteries, enhancing blood flow. Yet, these procedures are invasive, expensive, and carry risks like infection, bleeding, and restenosis. In light of these challenges, gene therapy has emerged as a potential alternative or complementary approach for CAD. Gene therapy targets the root causes and consequences of the disease at the molecular and cellular levels, focusing on four primary categories: angiogenic gene therapy, anti-inflammatory gene therapy, anti-apoptotic gene therapy, and anti-restenosis gene therapy.
Gene Therapy for Heart Failure
Heart failure (HF), another prevalent cardiovascular disorder, is characterized by the heart's inability to pump sufficient blood and oxygen to meet the body's demands. HF can arise from various factors such as myocardial infarction, hypertension, cardiomyopathy, and valvular disease, leading to damage to the heart muscle and impairment of its contractility, relaxation, and electrical activity. Current HF treatments involve medications like beta-blockers, angiotensin-converting enzyme inhibitors, and diuretics, aiming to reduce the heart's workload, lower blood pressure, and prevent fluid retention. However, these medications do not reverse structural and functional heart changes or halt disease progression. Mechanical devices, including implantable cardioverter-defibrillators, cardiac resynchronization therapy devices, and ventricular assist devices, offer solutions by correcting arrhythmias, enhancing ventricular synchronization, and supporting heart pumping function. Yet, these devices are invasive, costly, and associated with complications like infection, bleeding, and thrombosis. Gene therapy, therefore, emerges as a promising alternative or complementary therapy for HF, targeting the disease's underlying causes and consequences at the molecular and cellular levels. This approach encompasses four main categories: contractility-enhancing gene therapy, calcium-handling gene therapy, metabolic gene therapy, and regenerative gene therapy.
Gene Therapy for Vein Graft Stenosis
Vein graft stenosis (VGS) represents another significant cardiovascular disorder, resulting from the narrowing or blockage of vein grafts utilized to bypass obstructed coronary arteries during CABG procedures. VGS adversely impacts blood flow and graft patency, elevating the risk of complications like ischemia, angina, and myocardial infarction. Current treatments for VGS involve medications such as anti-platelet drugs, statins, and angiotensin receptor blockers, designed to prevent blood clots, lower cholesterol levels, and inhibit the renin-angiotensin system. However, these medications cannot reverse the pathological changes in vein grafts or impede disease progression. Surgical interventions, like repeat CABG or PCI, offer a means to restore blood flow and graft patency. Nonetheless, these procedures are invasive, expensive, and associated with complications such as infection, bleeding, and restenosis. Hence, gene therapy emerges as a promising alternative or complementary treatment for VGS, targeting the disease's root causes at the molecular and cellular levels. Gene therapy for VGS falls into four primary categories: anti-proliferative gene therapy, anti-inflammatory gene therapy, anti-thrombotic gene therapy, and endothelialization-enhancing gene therapy.
Table 1. Gene Therapy Approaches, Achievements, and Challenges for Diverse Cardiovascular Disorders
| Type of cardiovascular disorder | Type of gene therapy | Main achievements | Main challenges |
| Coronary artery disease (CAD) | Angiogenic gene therapy | Increased blood vessel formation and blood flow in ischemic myocardium | Variable and transient gene expression, lack of specificity and control, and limited clinical benefits |
| Anti-inflammatory gene therapy | Reduced inflammation and plaque formation in coronary arteries | Low delivery efficiency, potential immunogenicity and toxicity, and limited clinical trials | |
| Anti-apoptotic gene therapy | Reduced cardiomyocyte death and fibrosis in the ischemic myocardium | Low delivery efficiency, potential immunogenicity and toxicity, and limited clinical trials | |
| Anti-restenosis gene therapy | Reduced neointimal hyperplasia and restenosis in coronary arteries | Variable and transient gene expression, lack of specificity and control, limited clinical benefits | |
| Heart failure (HF) | Contractility-enhancing gene therapy | Increased cardiomyocyte contractility and cardiac function | Variable and transient gene expression, lack of specificity and control, potential arrhythmias |
| Calcium-handling gene therapy | Improved calcium cycling and cardiac function | Variable and transient gene expression, lack of specificity and control, potential arrhythmias | |
| Metabolic gene therapy | Enhanced energy production and cardiac function | Variable and transient gene expression, lack of specificity and control, potential metabolic imbalances | |
| Regenerative gene therapy | Induced cardiomyocyte proliferation and regeneration | Low efficiency and specificity, potential tumorigenicity and immunogenicity, limited clinical trials | |
| Vein graft stenosis (VGS) | Anti-proliferative gene therapy | Inhibited smooth muscle cell proliferation and neointimal hyperplasia in vein grafts | Variable and transient gene expression, lack of specificity and control, limited clinical benefits |
| Anti-inflammatory gene therapy | Suppressed inflammation and neointimal hyperplasia in vein grafts | Low delivery efficiency, potential immunogenicity and toxicity, and limited clinical trials | |
| Anti-thrombotic gene therapy | Prevented thrombus formation and occlusion in vein grafts | Variable and transient gene expression, lack of specificity and control, and potential bleeding complications | |
| Endothelialization-enhancing gene therapy | Promoted endothelial cell growth and function in vein grafts | Low delivery efficiency, potential immunogenicity and toxicity, and limited clinical trials |
Conlusion
In summary, gene therapy presents a promising avenue for addressing cardiovascular disorders, including CAD, HF, and VGS. By targeting the molecular and cellular mechanisms underlying these conditions, gene therapy can modulate gene expression or function in affected cells, tissues, or organs. Notably, gene therapy offers the advantage of long-term or permanent therapeutic effects, overcoming the limitations of conventional treatments, often palliative, transient, or accompanied by adverse effects. However, gene therapy encounters challenges and limitations, including the efficiency, specificity, and safety of gene vectors' delivery, immunogenicity, and toxicity of transgenes, regulation and stability of gene expression, and ethical and regulatory concerns regarding gene manipulation. Therefore, ongoing research and development efforts are crucial to optimize gene therapy strategies and protocols. Rigorous evaluation of their long-term efficacy and safety in larger and diverse populations is necessary, addressing potential risks and concerns associated with gene therapy applications. Gene therapy holds immense potential to revolutionize the treatment landscape for cardiovascular disorders, enhancing the quality of life and survival rates for millions of patients worldwide.
Reference
- Tabassum R, Ripatti S. Integrating lipidomics and genomics: emerging tools to understand cardiovascular diseases. Cell Mol Life Sci. 2021 Mar;78(6):2565-2584.

