Gene Therapy for Neurodegenerative Disorders
Inquiry NowNeurodegenerative disorders are a group of diseases that profoundly impact the structure and function of the nervous system, resulting in the progressive loss of neurons and the onset of cognitive, motor, and sensory impairments. Some of the most prevalent neurodegenerative disorders include Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, Huntington’s disease, and spinal muscular atrophy. These disorders impose a substantial burden on individuals, families, and society, as they diminish the quality of life, reduce life expectancy, and elevate healthcare costs. Despite extensive research efforts, the precise causes and mechanisms of neurodegeneration remain poorly understood, and effective cures or treatments for most of these disorders remain elusive. Current therapies primarily target symptom alleviation or the slowing of disease progression, but they are characterized by limited efficacy, adverse effects, and significant variability among individuals. Thus, there is an urgent imperative to develop novel and alternative approaches for the treatment of neurodegenerative disorders. One such promising approach is gene therapy, which has garnered significant attention in recent years. Gene therapy is a technique involving the introduction or modification of genetic material in cells to achieve therapeutic outcomes. Gene therapy boasts several advantages over conventional treatments for neurodegenerative disorders. It offers the ability to target specific genes or pathways implicated in the disease process, the potential to modify the disease course, and the capacity to prevent further damage. Moreover, gene therapy is compatible with other treatment modalities.
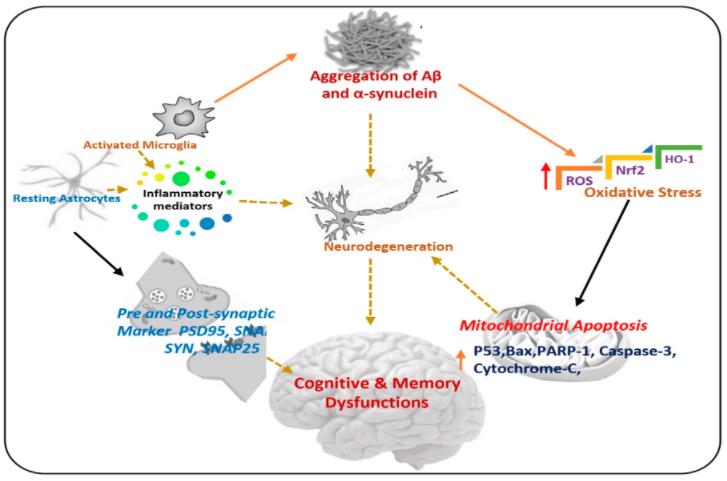 Fig.1 Pathogenesis of Neurodegenerative Diseases (Khan A, 2020)
Fig.1 Pathogenesis of Neurodegenerative Diseases (Khan A, 2020)
Features of Gene Therapy for Neurodegenerative Disorders
Gene therapy for neurodegenerative disorders possesses several distinctive features, rendering it a promising and attractive approach. One of its main features is its capacity to target specific cells or regions in the brain or spinal cord affected by the disease, such as neurons, glia, or neural stem cells. This specificity enhances the efficiency of gene delivery, reducing the risk of off-target effects or systemic toxicity. Additionally, gene therapy exhibits long-term or sustained effects, ensuring stable and durable expression of therapeutic genes or modulation of disease-related genes. This stability can potentially modify the disease course or prevent further damage, thereby reducing the need for repeated administration or maintenance therapy. Consequently, this enhances patient compliance and convenience. Another remarkable feature of gene therapy is its ability to synergize with other therapies, including drugs, surgery, or stem cell therapy, thereby achieving additive or synergistic effects. For instance, gene therapy can augment the survival or differentiation of transplanted stem cells and increase the sensitivity or responsiveness of cells to drugs. Furthermore, gene therapy enables the delivery of genes encoding neurotrophic factors, anti-inflammatory agents, antioxidants, or enzymes that protect or repair cells from oxidative stress, inflammation, or metabolic dysfunction. These features underscore gene therapy's versatility and flexibility, allowing tailoring to various types of neurodegenerative disorders and different disease progression stages.
Research and Clinical Progress of Gene Therapy for Neurodegenerative Disorders
In recent years, substantial progress has been made in gene therapy research and clinical trials for neurodegenerative disorders. These advancements demonstrate the therapy's potential effectiveness, safety, feasibility, and applicability. Representative studies are summarized in Table 1.
Table 1. Research and Clinical Progress of Gene Therapy for Neurodegenerative Disorders
| Disease | Vector | Gene | Delivery | Outcome |
| Alzheimer’s disease | AAV2-NGF | Nerve growth factor (NGF) | Intracerebral injection | Improved cognitive function and brain metabolism |
| Parkinson’s disease | AAV2-GAD | Glutamic acid decarboxylase (GAD) | Intraputaminal infusion | Improved motor function and quality of life |
| Amyotrophic lateral sclerosis | AAV9-SOD1-shRNA | Short hairpin RNA (shRNA) for superoxide dismutase 1 (SOD1) | Intraspinal injection | Reduced SOD1 levels in cerebrospinal fluid |
| Huntington’s disease | AAV5-miHTT | MicroRNA (miRNA) for mutant huntingtin (mHTT) | Intrastriatal injection | Improved motor function and neuropathology |
| Spinal muscular atrophy | Zolgensma (onasemnogene abeparvovec-xioi) | Survival motor neuron 1 (SMN1) | Intravenous infusion | Improved survival and motor milestones |
These studies offer compelling evidence of gene therapy's potential benefits and the challenges it presents in the context of neurodegenerative disorders. However, further research is essential to confirm the therapy's long-term effects, optimize dosages and delivery methods, and expand its indications and applications in the treatment of neurodegenerative disorders.
Reference
- Khan, Amjad, et al. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 9.7 (2020): 609.
