Immunotherapy
Immunotherapy, a groundbreaking cancer treatment, harnesses the body's immune system to combat cancer cells. In contrast to traditional methods like surgery, chemotherapy, and radiation, which directly attack cancer cells, immunotherapy focuses on boosting the immune system's ability to recognize and eliminate cancerous cells. This approach presents several advantages over conventional therapies, including specificity, durability, synergy, and reduced toxicity. Despite its promises, immunotherapy encounters challenges such as resistance, variability, cost, and accessibility. The roots of immunotherapy trace back to the late 19th century when William Coley observed tumor regression in patients with bacterial infections. Since then, diverse immunotherapies have been developed, including monoclonal antibodies, checkpoint inhibitors, cancer vaccines, CAR T-cell therapy, and others. Each type boasts distinct mechanisms, indications, outcomes, and side effects.
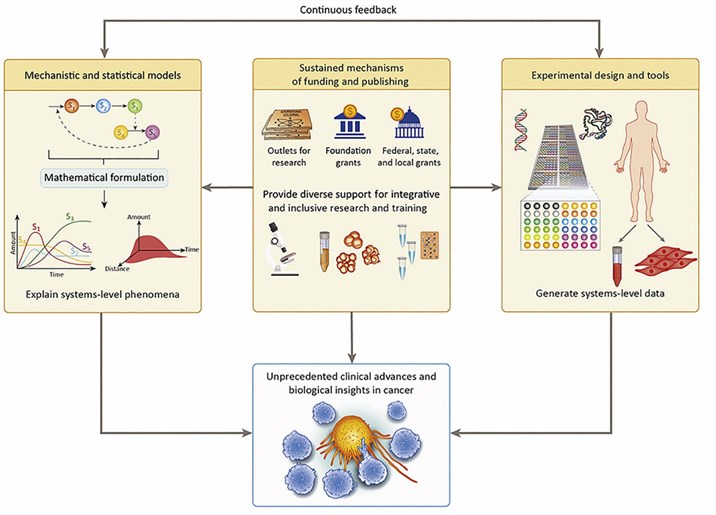 Fig.1 The keys to Success for Systems Approaches to Cancer Immunotherapy (Szeto GL, 2019)
Fig.1 The keys to Success for Systems Approaches to Cancer Immunotherapy (Szeto GL, 2019)
Advantages of Immunotherapy for Cancer
Immunotherapy for cancer offers several distinct advantages, making it a revolutionary approach in the fight against this disease. One of its key features is specificity. Immunotherapy can precisely target and eliminate cancer cells while sparing normal, healthy cells. This precision is achieved by leveraging the immune system's ability to differentiate between self and non-self antigens, triggering an immune response. Cancer cells often display abnormal or mutated antigens, signaling danger to the immune system. Utilizing immunotherapy methods such as monoclonal antibodies or CAR T-cell therapy, these antigens are targeted and bound by specific receptors or antibodies. Consequently, the immune system is activated to annihilate the cancer cells, reducing the risk of harming normal tissues—a common side effect of conventional treatments like chemotherapy and radiation. Another pivotal advantage of immunotherapy is its durability. This treatment can provide long-lasting protection against cancer recurrence or metastasis by inducing immunological memory. Immunological memory empowers the immune system to recognize and respond rapidly and robustly to previously encountered antigens. Immunotherapies like cancer vaccines or checkpoint inhibitors can train the immune system, stimulating the production of enduring immune cells, including memory T cells or B cells. These cells persist within the body, ready to mount a swift and effective response against any residual or new cancer cells that may emerge. Additionally, immunotherapy demonstrates synergy when combined with other therapies or agents. By modulating the immune system's response to cancer cells, immunotherapy enhances the sensitivity and effectiveness of other treatments. Techniques such as checkpoint inhibitors or cytokines play a crucial role in this synergy. They can block inhibitory signals or boost stimulatory signals within the immune system, overcoming the resistance or tolerance that cancer cells often develop against other therapies or agents.
Research and Clinical Advancements
Immunotherapy for cancer represents a dynamic and swiftly evolving realm within both research and clinical practice. Across a myriad of cancers, numerous studies and trials have been diligently conducted to assess the efficacy and safety of immunotherapy. Recent findings from the World Health Organization underscore the sheer magnitude of this effort: there are over 3,000 ongoing clinical trials worldwide, encompassing more than 500,000 patients. The outcomes from these trials have been nothing short of remarkable, demonstrating the potential of immunotherapy to yield substantial benefits. Patients have experienced complete or partial responses, prolonged survival, and an enhanced quality of life. One such breakthrough occurred in a phase III trial involving pembrolizumab, a checkpoint inhibitor disrupting the PD-1/PD-L1 pathway, in patients with advanced non-small cell lung cancer (NSCLC). Regardless of their PD-L1 expression levels, patients treated with pembrolizumab exhibited significantly improved overall survival and progression-free survival compared to those undergoing chemotherapy. In another compelling instance, a phase II trial focused on axicabtagene ciloleucel, a CAR T-cell therapy targeting the CD19 antigen, in patients with refractory large B-cell lymphoma. Axicabtagene ciloleucel yielded outstanding results, boasting an overall response rate of 82% and a complete response rate of 54%, with responses lasting a median of 11.1 months. Despite these successes, it's crucial to acknowledge that not all patients or cancers respond uniformly to immunotherapy. Some may exhibit resistance or refractoriness, while others might encounter severe adverse events. Identifying predictive factors for response and toxicity is paramount. Factors like biomarkers, tumor microenvironment, immune status, and combination therapies play pivotal roles in this regard. Moreover, the field continues to evolve, with ongoing efforts directed toward overcoming challenges and limitations. Researchers are exploring novel targets, inventive delivery systems, advanced engineering methods, and sophisticated monitoring tools.
Table 1. Examples of Immunotherapy for Cancer in Clinical Trials
| Type of immunotherapy | Target | Trial | Outcome |
| Pembrolizumab (checkpoint inhibitor) | PD-1/PD-L1 pathway | Phase III trial in advanced NSCLC | Improved overall survival and progression-free survival |
| Axicabtagene ciloleucel (CAR T-cell therapy) | CD19 antigen | Phase II trial in refractory large B-cell lymphoma | Induced high response rate and durable response |
References
- Liu C, et al. Clinical cancer immunotherapy: Current progress and prospects. Front Immunol. 2022 Oct 11;13:961805.
- Sharma P, et al. The future of immune checkpoint therapy. Science. 2015 Apr 3;348(6230):56-61.
- Szeto GL, Finley SD. Integrative Approaches to Cancer Immunotherapy. Trends Cancer. 2019 Jul;5(7):400-410.
- Carter PJ, et al. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2020 Aug;19(8):533-548.
- Reck M, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016 Nov 10;375(19):1823-1833.
- Schuster SJ, et al. Axicabtagene ciloleucel in relapsed or refractory large B-cell lymphoma. N Engl J Med. 2019 Jan 3;380(1):45-56.
- Ribas A, et al. PD-L1 blockade with durvalumab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018 Jul 26;379(4):341-351.
- Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug 19;363(8):711-23.
- Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020 May 14;382(20):1894-1905.
- Le DT, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015 Jun 25;372(26):2509-20.
