Oncolytic Virotherapy
Oncolytic virotherapy is a form of gene therapy that utilizes viruses to infect and eliminate cancer cells. In contrast to conventional therapies such as surgery, chemotherapy, and radiotherapy, which can harm normal tissues and lead to severe side effects, oncolytic virotherapy offers a more targeted, safe, and effective approach to eradicate malignant tumors. Additionally, oncolytic virotherapy can stimulate the host immune system to recognize and eliminate any remaining cancer cells, thus preventing tumor recurrence and metastasis. Furthermore, oncolytic virotherapy can be synergistically combined with other treatments to enhance their effects and overcome their limitations.
Oncolytic viruses, either naturally occurring or genetically modified, have the ability to selectively replicate within and lyse cancer cells while sparing normal cells. Several types of oncolytic viruses have been studied for cancer treatment, including adenovirus, herpes simplex virus, measles virus, reovirus, and vaccinia virus. Each type of oncolytic virus has its own advantages and disadvantages, depending on characteristics such as genome structure, replication cycle, pathogenicity, and immunogenicity. Therefore, the selection of the most suitable oncolytic virus for a specific type of cancer is a critical step in the development of oncolytic virotherapy.
Key Features of Oncolytic Virotherapy
There are three main features of oncolytic virotherapy: self-amplification, multimodality, and versatility. First, oncolytic virotherapy is self-amplifying, meaning that the oncolytic viruses can replicate and spread within the tumor, increasing the therapeutic dose and coverage. This property can address the challenges of low viral delivery and poor tumor penetration, which can limit the effectiveness of other gene therapies. Self-amplification can also reduce the cost and frequency of administration while enhancing the safety and efficacy of oncolytic virotherapy. Second, oncolytic virotherapy is multimodal, enabling it to achieve multiple therapeutic effects simultaneously, including direct cytotoxicity, immune activation, gene delivery, and combination therapy. This capability can improve the overall outcome and durability of oncolytic virotherapy, overcoming the heterogeneity and resistance often encountered in tumors that reduce the effectiveness of other therapies. Multimodality also provides greater flexibility and versatility for designing and optimizing oncolytic virotherapy. Third, oncolytic virotherapy is versatile, making it applicable to various types of cancers, regardless of their origin, location, stage, or mutation status. This versatility expands the scope and applicability of oncolytic virotherapy, addressing the unmet needs and challenges in cancer treatment that are not resolved by other therapies. It also enables the development of more personalized and precise oncolytic virotherapy for different patients and tumor types.
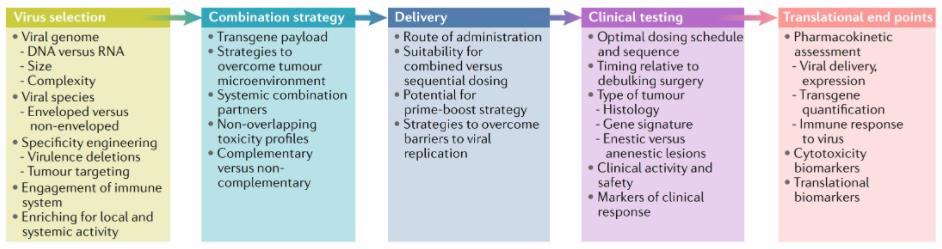 Fig.1 Considerations for OV drug development as part of the immune-oncology toolbox
Fig.1 Considerations for OV drug development as part of the immune-oncology toolbox
Research and Clinical Advancements in Oncolytic Virotherapy
Oncolytic virotherapy stands as one of the most active and promising domains in global cancer research and development. Recent reports reveal over 300 oncolytic virotherapy projects in diverse stages of development, encompassing over 100 oncolytic viruses and addressing more than 20 types of cancers. The global market for oncolytic virotherapy is anticipated to reach $1.5 billion by 2027, reflecting a remarkable compound annual growth rate of 26.5%. This substantial growth can be attributed to various factors, including the rising incidence and prevalence of cancer, the persisting gaps in current therapies, advancements in biotechnology and virology, and the supportive initiatives from regulatory bodies and funding organizations.
Oncolytic virotherapy has demonstrated promising outcomes in both preclinical and clinical studies across a spectrum of cancer types. Table 1 provides a summary of these studies, illustrating the breadth of research in the oncolytic virotherapy field.
Table 1. Preclinical and Clinical Studies of Oncolytic Virotherapy
| Type of cancer | Oncolytic virus | Genetic modification | Combination therapy | Preclinical or clinical results |
| Melanoma | Talimogene laherparepvec (T-VEC) | Herpes simplex virus expressing GM-CSF | Ipilimumab or pembrolizumab | Tumor regression and immune activation in patients with advanced melanoma |
| Glioma | DNX-2401 | Adenovirus expressing IL-12 | Pembrolizumab | Favorable safety profile and significant survival benefit in patients with recurrent GBM |
| Prostate cancer | CG0070 | Adenovirus expressing GM-CSF | Docetaxel | High response rate and durable antitumor effect in patients with NMIBC or mCRPC |
| Breast cancer | ONCOS-102 | Adenovirus expressing GM-CSF | Chemotherapy, radiotherapy, or immunotherapy | Synergistic effects with other therapies in preclinical models of breast cancer and clinical trials of ovarian cancer, mesothelioma, and melanoma |
| Pancreatic cancer | Pexa-Vec | Vaccinia virus expressing GM-CSF | Chemotherapy | Remarkable efficacy in preclinical models of pancreatic cancer and clinical trials of HCC, RCC, and CRC |
References
- Russell SJ, et al. Oncolytic virotherapy. Nat Biotechnol. 2012 Jul;30(7):658-70.
- Kaufman HL, et al. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015 Sep;14(9):642-62.
- Hemminki O, et al. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol. 2020 Jun 29;13(1):84.
- Harrington K, et al. Optimizing oncolytic virotherapy in cancer treatment. Nat Rev Drug Discov. 2019 Sep;18(9):689-706.
- Mondal M, et al. Recent advances of oncolytic virus in cancer therapy. Hum Vaccin Immunother. 2020 Oct 2;16(10):2389-2402.
- Wennier S, et al. Oncolytic virotherapy for pancreatic cancer. Expert Rev Mol Med. 2011 May 18;13:e18.
- Breitbach CJ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011 Aug 31;477(7362):99-102.
- Heo J, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013 Mar;19(3):329-36.
- Andtbacka RH, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J Clin Oncol. 2015 Sep 1;33(25):2780-8.
- Lang FF, et al. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J Clin Oncol. 2018 May 10;36(14):1419-1427.
- Kaufman HL, et al. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015 Sep;14(9):642-62.
- Breitbach CJ, et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature. 2011 Aug 31;477(7362):99-102.
