HPLC-MS/MS-based Linearity Assessment Service
Linearity assessment is crucial in determining the ability of an analytical method to produce results that are directly proportional to the concentration of analytes within a specified range. Creative Biolabs is renowned for broad drug development service capabilities, including bioanalytical testing using advanced high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) technologies. We provide comprehensive support for method validation, including linearity assessment.
Superiority of HPLC-MS/MS-based Linearity Assessment
HPLC-MS/MS-based linearity assessment stands out due to its superior sensitivity, selectivity, accuracy, and versatility.Here are the key reasons why HPLC-MS/MS-based linearity assessment is preferred and considered superior:
-
HPLC-MS/MS can detect analytes at very low concentrations.
-
The tandem mass spectrometry portion of HPLC-MS/MS provides excellent selectivity by combining chromatographic separation and specific mass detection.
-
HPLC-MS/MS offers precise and accurate quantification of analytes due to its ability to measure multiple reaction products simultaneously.
-
HPLC-MS/MS can cover a broad range of analyte concentrations within a single method.
-
HPLC-MS/MS supports robust method validation protocols, including linearity assessment, as part of regulatory requirements.
HPLC-MS/MS-based Linearity Assessment Service at Creative Biolabs
Creative Biolabs' HPLC-MS/MS-based linear assessment service is used to support quantitative analysis studies in biological matrices. Linearity and limit of quantification (LOQ) are assessed by spiking test compounds at concentrations in a biological matrix and then quantifying using the specified sample preparation and analytical methods.
Prepare Standard Solutions
Prepare a range of standard solutions for analytes with known concentrations.
Chromatographic Separation & Detection
The analytes are separated from the other components in the sample by the HPLC system.
Data Collection
Record the peak area or height of each standard solution.
Calibration Curves
The peak area or peak height is plotted against the known concentration of the standard to create a calibration curve.
Linearity Evaluation
Linearity is evaluated by checking the calibration curve. The degree to which the data fit the straight line is determined by calculating the correlation coefficient (R²). An R² value close to 1 indicates good linearity.
Detailed Information of Our Service
Creative Biolabs has expertise in data analysis to help you properly interpret calibration curves and linear results. The following is biological and assay information of our linear assessment service:
Data Display
The figure below illustrates the dilution linearity and correlation analysis of different samples using LC-MS/MS. (A) Dilution linearity. (B) Relevance.
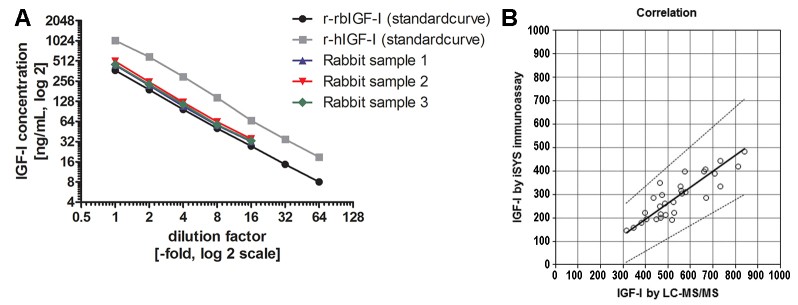 Fig.1 LC-MS/MS-based linearity and correlation analysis.1
Fig.1 LC-MS/MS-based linearity and correlation analysis.1
Main Applications of HPLC-MS/MS-based Linearity Assessment
HPLC-MS/MS-based linearity assessment is a key component of analytical method validation in a variety of fields, including pharmaceutical analysis, clinical diagnostics, environmental monitoring, forensics, and food safety. Here are the key applications of HPLC-MS/MS-based linearity assessment:
Frequently Asked Question
A1: How to ensure the accuracy of linear assessment?
Q1: Ensuring the accuracy of linearity assessment in HPLC-MS/MS requires meticulous attention to detail at every step of the process, from the preparation of calibration standards to the statistical verification of results. Creative Biolabs helps you achieve reliable and accurate linear assessments by following these guidelines.
If you are interested in In Vitro ADME assays, please take a look at the following services:
Contact Us
If you would like to discuss your specific needs about linear assessment based on HPLC-MS/MS method, please don't hesitate to contact us.
Reference
-
Bielohuby, Maximilian et al. "Validation of serum IGF-I as a biomarker to monitor the bioactivity of exogenous growth hormone agonists and antagonists in rabbits." Disease models & mechanisms vol. 7,11 (2014): 1263-73.
For Research Use Only | Not For Clinical Use



 Fig.1 LC-MS/MS-based linearity and correlation analysis.1
Fig.1 LC-MS/MS-based linearity and correlation analysis.1

 Download our brochure
Download our brochure