Phage display technology, allowing the construction of libraries in which various peptides and proteins are displayed on the phage, has widespread applications.
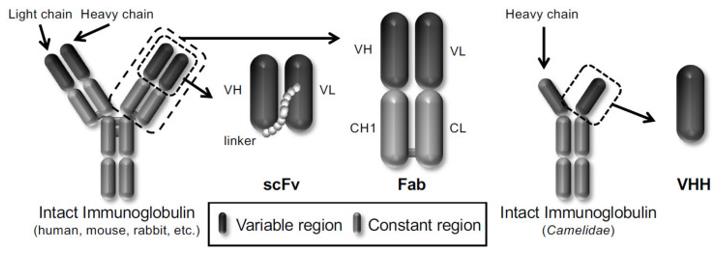 Fig 1. Schematic representation of different types of antibody formats displayed on the phages. (Kazuya, et al. 2021)
Fig 1. Schematic representation of different types of antibody formats displayed on the phages. (Kazuya, et al. 2021)
The use of hybridomas to manufacture monoclonal antibodies was invented in 1975. Because of their great specificity and affinity for the target molecule, monoclonal antibodies have been considered a possible "panacea," but clinical studies have proven disappointing. There are several causes of failure, many of which can be addressed.
Gene libraries, constructed using mutations in antigen-binding regions such as VL and VH, are displayed on phage, and antibodies with high affinity for the antigen are then isolated by biopanning.
Methods for constructing gene libraries with mutations:
Despite their distinct and superior properties, antibody drugs' targets are dwindling. As a result, identifying prospective targets for antibody drugs is critical. The use of phages to identify possible therapeutic targets is an efficient method.
Autoantibodies to autoantigens can be employed as biomarkers in autoimmune disorders. The search for antigens that bind to autoantibodies in autoimmune diseases is a potential strategy for elucidating molecular pathophysiology and target discovery, and phage display techniques can be useful in studying autoimmune-specific autoantigens.
Antibody proteomics technology isolates monoclonal antibodies to candidate proteins using phage antibody libraries and TMA analysis to find potential biomarkers and therapeutic targets.
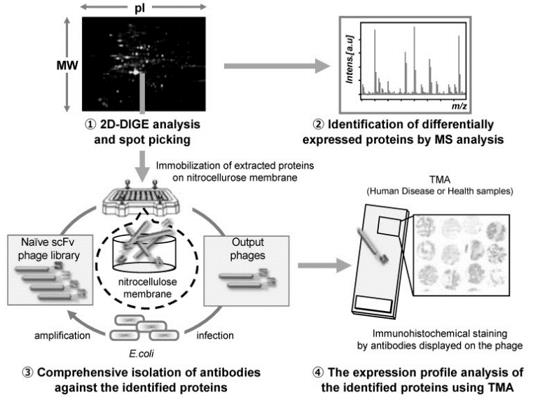 Fig 2. Schematic illustration of the antibody proteomics system. (Kazuya, et al. 2021)
Fig 2. Schematic illustration of the antibody proteomics system. (Kazuya, et al. 2021)
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.