Creative Biolabs is committed to provide advanced tools for scFv/Fab expression and purification. As a leading supplier for reagents in the biotechnology field, we understand the importance of convenient and easy-to-use systems for high level expression and sample purification. We invite you to review our growing range of expression systems resulting from our experience in cloning, overexpression and purification.
scFv/Fab Production in Bacterial Cells
We have a series of well-tested bacterial expression systems established in house for high-yield and highly active scFv/Fab production. In particular, we have a well-established SUMO Technology for scFv and Fab production in bacterial cells, which allows high level expression of scFv and Fab antibodies with binding activity without tag.
• Expression strategies:
Secreting expression in periplasm of E. coli.
Soluble expression in cytoplasm of E. coli.
Insoluble expression as inclusion bodies.
scFv production through pET expression system.
Fab production using pCDisplay phagemid vector.
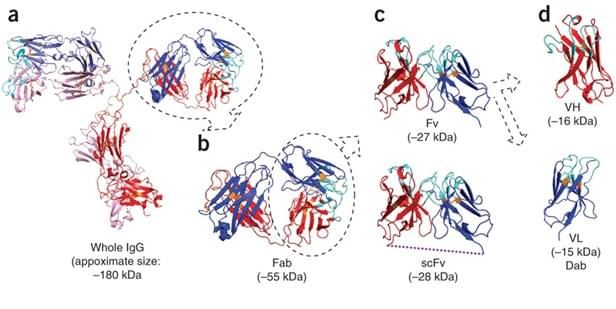 Fig. 1 Schematic representation of various antibody formats. (Tanaka and Rabbitts 2010)
Fig. 1 Schematic representation of various antibody formats. (Tanaka and Rabbitts 2010)
scFv/Fab Production in Mammalian Cells
Of note, our combined expertise in antibody engineering and mammalian cell based bio-manufacturing allows us efficiently streamlining the production of recombinant scFv/Fab and bivalent scFv/Fab using transient or stable transfection in mammalian cells. We have established a solid platform for industrial scale production of recombinant scFv/Fab in mammalian cells. We constructed a series of mammalian expression vectors that are specialized in scFv or Fab production. In the end of production, the recombinant antibody will be three-step purified (protein A/G followed by two polishing steps, antibody monomer >99%, endotoxin level >0.5 EU/mg antibody, protein A residual < 10 PPM, which is equivalent to antibody for human use). Our head in charge of this recombinant antibody production business received a Ph.D. from MIT and had over 6 year’s industrial experience at Merck Research Lab at West Point.
Reference
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
