The peroxisome proliferator-activated receptors (PPARs) are ligand-inducible transcription factors that belong to the superfamily of phylogenetically related proteins termed as nuclear receptors. Three different PPAR isotypes (PPARα, PPARβ/δ, and PPARγ) have been identified in various species. PPARs play an important role in the pathogenesis of various disorders of the CNS. Creative Biolabs is dedicated to establishing the most exquisite service platform for our clients and providing one-stop nuclear receptors modeling services to support your projects.
PPAR agonists have shown efficacy in Parkinson's disease, Alzheimer's disease, and brain injury. They act on microglial cells and inhibit microglial cells’ activation. The role of PPARs in modulating lipid and glucose metabolism is well established. More recently, PPARs have been demonstrated to modulate inflammation. For example, PPAR agonists inhibit the production of proinflammatory molecules by peripheral immune cells as well as resident glial cells. Furthermore, PPAR agonists have proven effective in suppressing the development of animal models of CNS inflammatory and neurodegenerative disorders. In vivo oral administration of the PPARγ agonist - pioglitazone reduced glial activation and the accumulation of Aβ-positive plaques in the hippocampus and cortex. Various neurodegenerative diseases are associated with electron transport chain enzyme activity reductions and increased mitochondrial-generated oxidative stress.
Generally, the PPAR binding sites consist of three arms: arm I exhibits mainly polar character and includes the AF-2 helix that contains the transcriptional activation domain. The hydrophilic head group of the PPAR agonist interacts with this part of the pocket, whereas the hydrophobic tail of the PPAR ligand is either buried in the hydrophobic arm II or arm III that is formed by hydrophobic and hydrophilic residues. The ligand-binding domains (LBDs) of the three PPAR subtypes are very large and bind a multitude of natural and synthetic ligands. PPARα has the largest and most hydrophobic pocket, followed by PPARγ and PPARβ/δ. Moreover, the PPARα ligand-binding domain seems to be predestined for binding hydrophobic fatty acids, whereas the more polar PPARγ pocket favors hydroxylated fatty acid ligands. The pocket of PPARβ/δ differs substantially from the LBDs of PPARα and PPARγ, especially in the area next to the activation function helix 2. It cannot accommodate large hydrophobic groups linked to the agonist’s hydrophilic head group because of its small size.
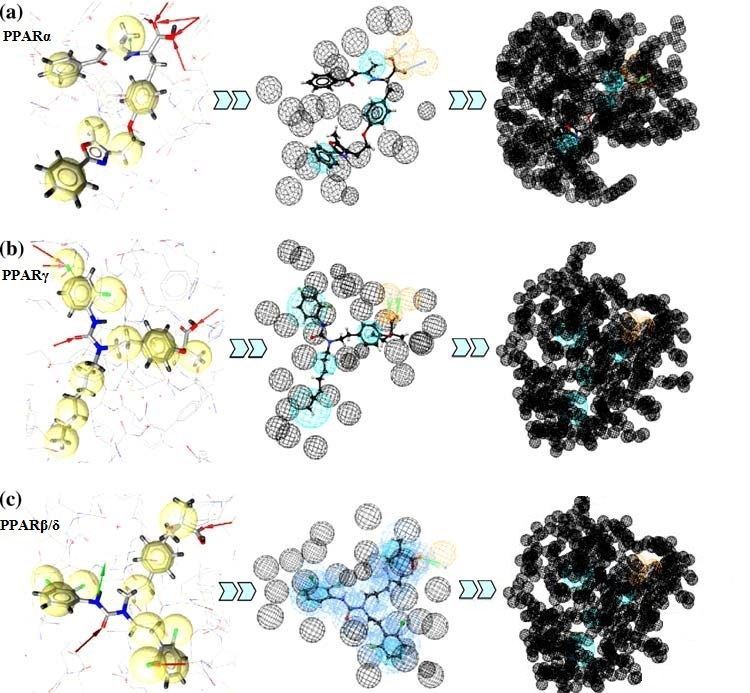 Fig.1 Generation of the structure-based PPAR agonists model.
Fig.1 Generation of the structure-based PPAR agonists model.
Diseases of the CNS present a challenge for the development of new therapeutic agents. Nuclear receptors have proven to be valuable targets for the development of new drugs owing to their ability to directly regulate gene expression. The nuclear receptor, PPARγ, has been investigated for its action in ameliorating the development and progression of a number of CNS diseases. PPARγ agonists exhibit potent anti-inflammatory effects and appear to have direct neuroprotective actions. PPARγ agonists have been shown to be efficacious in animal models of Alzheimer’s disease, Parkinson's disease, multiple sclerosis. We can provide a variety of structure-based nuclear receptors modeling approaches to meet customers’ specific requirements.
Creative Biolabs has focused on the development of computational protein design for years, so we whole-heartedly cooperate with you to accomplish our shared goals. Our team provides you with outstanding support and meets your specific needs. If you are interested in our services, please contact us for more details.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.