Creative Biolabs is proud to introduce a unique therapeutic antibody discovery service: Humanization of Bovine Ultralong CDR3 Antibodies.
By merging the ultralong CDR3s discovered in bovine with human IgG scaffolds, novel therapeutic antibodies against challenging hot antigen families, including GPCRs and other intra-membrane targets on the cell surface are possible. Humanized IgG scaffolds that accommodate ultralong CDR3s may target small proteins with desirable therapeutic properties, which are challenging for conventional antibodies.
Evidence has shown that antibodies with unusually long CDR H3 regions may be a more effective defense against disease than typical antibodies. In cattle, 10% of antibodies have such ultralong CD3 H3 regions. Deep sequencing reveals a remarkable complexity of cysteines. Correspondingly, crystal structure reveals these CDR H3s form a very unusual architecture composed of a β strand "stalk" that supports a structurally diverse, disulfide-bonded "knob" domain, that can be elicited to recognize defined antigens. Through combinations of somatically generated disulfides, these ultralong CDR H3s can fold into a diversity of minidomains. These cow antibodies look interesting for their potential to zero on those targets that haven’t been successfully targeted by traditional antibodies, for example, the GPCRs, that have been intractable for antibody therapeutic development in the past.
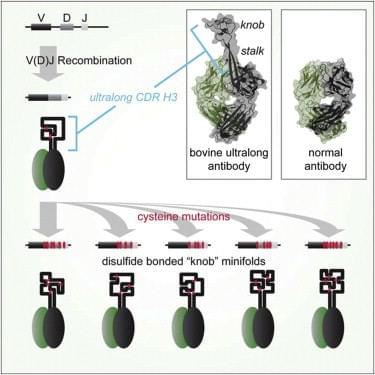 Fig. 1 Model for Ultralong CDR H3 Diversification into Minifolds. (Wang et al. 2013)
Fig. 1 Model for Ultralong CDR H3 Diversification into Minifolds. (Wang et al. 2013)
Besides developing therapeutic monoclonal antibodies with ultralong CDR3s, Creative Biolabs has also established a platform to raise bovine monoclonal antibodies with ultralong CDR3s.
Other optional Human or Humanized Antibody Services:
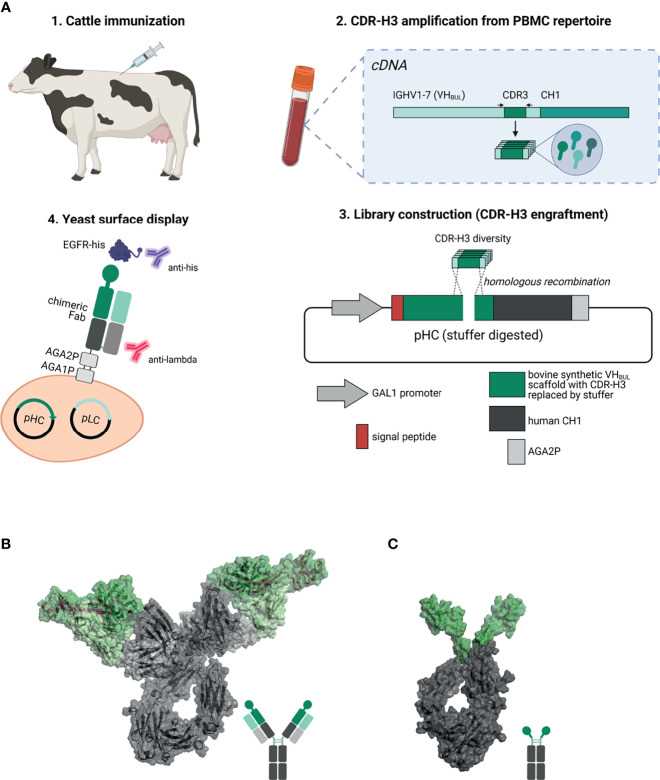 Fig. 2 Overview about the generation of EGFR-specific ultralong CDR-H3 antibodies and Knobbodies. (Lukas Pekar, 2021)
Fig. 2 Overview about the generation of EGFR-specific ultralong CDR-H3 antibodies and Knobbodies. (Lukas Pekar, 2021)
Here, the researchers combined bovine immune technology with yeast surface display technology to produce epidermal growth factor receptor (EGFR) specific bovine ultralong CDR-H3 antibodies. After immunization, ultra-long CDR-H3 regions were specifically amplified and transplanted into IGHV1-7 scaffolds by homologous recombination to promote Fab display. Antigen-specific clones were obtained by FACS and reformatted into chimeric antibodies. The results show that bovine chimeric antibodies can effectively induce antibody-dependent cell-mediated cytotoxicity (ADCC) and resist tumor cells with overexpression of EGFR. In addition, most antibodies can significantly inhibit EGFR-mediated downstream signal transduction. Scientists have also shown that a minor fraction of CDR-H3 knobs derived from the generated antibodies can act independently as complementary sites to promote EGFR binding when grafted into the Fc part of human IgG1.
Humanization of bovine ultralong CDR3 antibodies involves modifying the antibody derived from cattle so that it is less likely to be recognized as foreign by the human immune system. This process is essential for therapeutic applications, as it reduces the risk of immune responses that can diminish the efficacy of the antibody or lead to adverse reactions in humans. Bovine ultralong CDR3 antibodies are particularly valued for their unique ability to bind to antigenic targets that might be inaccessible to human antibodies, making them promising candidates for addressing complex diseases such as cancer and autoimmune disorders.
The humanization of bovine ultralong CDR3 antibodies is typically achieved through CDR grafting. This method involves transplanting the Complementarity-Determining Regions (CDRs) from the bovine antibody into a human antibody framework. These CDRs are the parts of the antibody that directly bind to antigens. By grafting them onto a human antibody framework, researchers can maintain the unique antigen-binding properties of bovine antibodies while significantly reducing their immunogenicity in humans. Additional refinements, such as modifying certain framework residues to better support the bovine CDRs, can further enhance stability and binding efficiency.
One of the primary challenges in humanizing bovine ultralong CDR3 antibodies is maintaining the original high specificity and affinity for antigens after modification. Bovine ultralong CDR3 regions often adopt unique and complex structures that are crucial for their ability to recognize and bind to specific targets. Transferring these regions into a human antibody framework without losing functional integrity requires precise engineering. Additionally, ensuring that the humanized antibodies do not elicit an immune response in humans adds another layer of complexity, as even minor discrepancies in the protein structure can trigger immunogenicity.
Recent advances in computational biology and protein engineering have significantly improved the success rates of humanizing bovine ultralong CDR3 antibodies. Techniques such as molecular modeling and in silico simulations are now commonly used to predict the structural compatibility of bovine CDRs with human frameworks, allowing for more accurate design of humanized antibodies. Furthermore, advancements in gene editing technologies, such as CRISPR/Cas9, enable precise modifications at the DNA level, facilitating the insertion of bovine CDR sequences into human antibody genes. These technological developments have streamlined the humanization process, increasing the feasibility of producing effective and safe bovine-derived therapeutic antibodies for clinical use.
Humanized bovine ultralong CDR3 antibodies are especially effective in applications where high specificity and affinity are critical, such as in targeting hidden or conformationally complex epitopes that are not easily accessed by human antibodies. These unique characteristics make them particularly useful in oncology, where they can be used to target cancer cells expressing specific, often cryptic, tumor antigens without affecting healthy cells. Additionally, due to their high specificity, these antibodies are also valuable in research and diagnostic settings, enabling precise detection and study of biomarkers associated with various diseases.
Bovine ultralong CDR3 regions are distinguished by their exceptional length and complexity compared to their human counterparts. Typically, CDR3 regions in human antibodies consist of about 12 to 15 amino acids, whereas in bovine antibodies, these regions can extend up to 60 amino acids. This extraordinary length allows bovine ultralong CDR3s to form unique structural motifs such as extended loops or even knob-like structures that can penetrate deep into antigenic sites that are generally inaccessible to human antibodies. This structural uniqueness is what makes bovine ultralong CDR3s highly desirable for targeting complex antigens, though it also presents challenges in maintaining structural integrity and function when transplanted into a human antibody scaffold during humanization.
High-throughput sequencing and advanced bioinformatics tools allow for a more precise understanding and selection of bovine antibodies with desirable properties for humanization. Structural biology technologies, such as X-ray crystallography and cryo-electron microscopy, provide detailed insights into the three-dimensional structures of these antibodies, facilitating more accurate modeling and engineering. Additionally, automated synthetic biology platforms are increasingly used to construct and test multiple variants of humanized antibodies rapidly, significantly accelerating the development process and improving the chances of retaining the original antibody's efficacy and specificity in the humanized version.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.