All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Immunotherapy has revolutionized cancer treatment, offering personalized and potent therapeutic options. However, challenges remain, such as high manufacturing costs and scalability issues associated with genetically modified therapies. To address these, Creative Biolabs introduces an innovative approach with our Allogeneic Antibody-Cell Conjugation (ACC) Cell Development Service. This cutting-edge platform leverages the principles of ACC technology to create potent, off-the-shelf cell therapies by conjugating tumor-targeting antibodies directly onto the surface of immune cells.
Creative Biolabs offers a comprehensive development service for allogeneic ACC cells, designed to provide efficient, scalable, and safe cellular therapies. By utilizing our proprietary ACC technology, we eliminate the necessity for genetic modifications, thereby reducing production complexities and associated costs. Our ACC cell development services are harnessed to fortify innate immune cells, such as natural killer (NK) cells and gamma delta (γδ) T cells, with tumor-targeting antibodies. This innovative approach offers superior cellular therapies that can recognize and eliminate cancer cells without the drawbacks of genetic modifications.
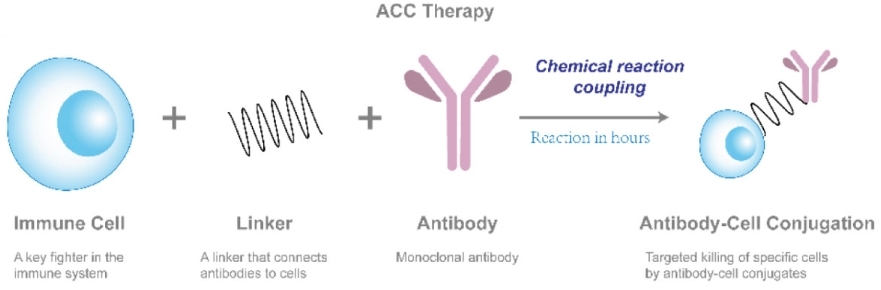 Fig.1 Schematic of ACC technology.1
Fig.1 Schematic of ACC technology.1
Creative Biolabs is dedicated to developing a one-stop service for allogeneic antibody-cell coupling cell development, where we provide comprehensive and rigorous detail control at every step involved.
Our ACC technology employs a straightforward chemical coupling method to attach tumor-targeting antibodies to cell surface proteins. Unlike chimeric antigen receptor T (CAR-T) cell therapy, ACC does not require time-intensive genetic modifications, thereby significantly reducing the manufacturing timeline and costs.
The ACC technology at Creative Biolabs is designed to provide high affinity and specificity. The covalent bonding of aptamers ensures sustained high affinity of antibody-conjugated effector cells towards tumor cells, leveraging immune activation receptors and activating signaling pathways such as NFAT.
The "plug and play" nature of ACC technology allows any antibody new or existing to be conjugated to the stimulatory surface receptors of our allogeneic immune cells. This modularity extends the application of ACC technology beyond oncology, potentially benefitting various immunological indications.
Preclinical studies provide compelling evidence of the efficacy of ACC cells. In vivo, these ACC cells exhibited potent anti-lymphoma activity in xenograft mouse models, prolonging survival and showing significantly enhanced cytotoxicity compared to non-conjugated cell therapies. For instance, ACE1831, a CD20-targeting γδ T cell product, showed superior performance against Rituximab-resistant lymphoma models, emphasizing its robust clinical potential.
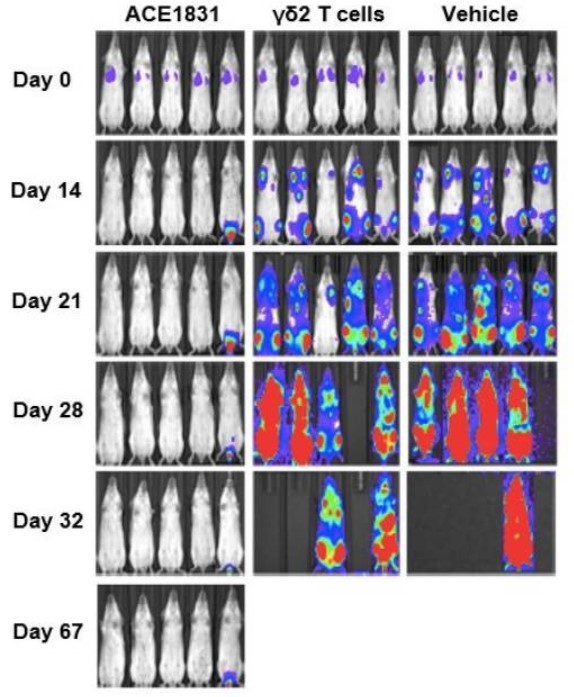 Fig.2 The superior potency of ACE1831 against CD20-expressing cancer cells in vivo.2
Fig.2 The superior potency of ACE1831 against CD20-expressing cancer cells in vivo.2
Q1: What types of cancers can ACC technology target?
A1: ACC technology is versatile and can be tailored to target a variety of hematological malignancies and solid tumors. Our CD20-targeting and HER2-targeting ACC products exemplify its broad applicability.
Q2: How does ACC technology compare to traditional CAR-T therapy?
A2: Unlike CAR-T therapy, which requires time-consuming and costly genetic modification, ACC technology employs a rapid chemical conjugation process. This not only reduces production time and costs but also minimizes potential safety concerns associated with genetic modifications.
Q3: What are the safety considerations of ACC technology?
A3: ACC technology ensures high specificity and selectivity of antibody binding, significantly reducing off-target effects. The stable conjugation of antibodies to immune cells provides reliable therapeutic performance while maintaining the safety profile.
Creative Biolabs' Allogeneic Antibody-Cell Conjugation Cell Development Service represents a significant advancement in cancer immunotherapy. By leveraging the unique features of ACC technology, we provide a robust, scalable, and cost-effective solution for the development of powerful anti-cancer therapies.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION