All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs offers customers superior approaches for scFv generation such as the one-stop solution for developing CAR from Hybridoma or Phage Display, which manifest as mature and delicate technologies for scFv construction. Particularly, a CAR-T Library Technology on our platform makes great contribution to the selection of desired scFv, whose expression and function are correlated with T cell activation activity.
Basically, a variable region (Fv) consists of a heavy chain variable region (VH) and a light chain variable region (VL), while a single-chain variable fragment (scFv) normally consists of a VH and a VL connected together via a peptide linker. As the most critical initial step, the generation of specific scFv and optimization of its superior affinity lay down the most basic foundation of an effective and specific CAR-T therapy. Without a doubt, a superior scFv leads to an even more superior CAR-T therapy. Creative Biolabs manifests as a world leading expert in antibody engineering field and provides our clients with the best scFv with the most superior affinity in order to meet their research needs. Currently, we offer the two strategies to generate scFv.
A. Generate scFv from Hybridoma Cell Lines
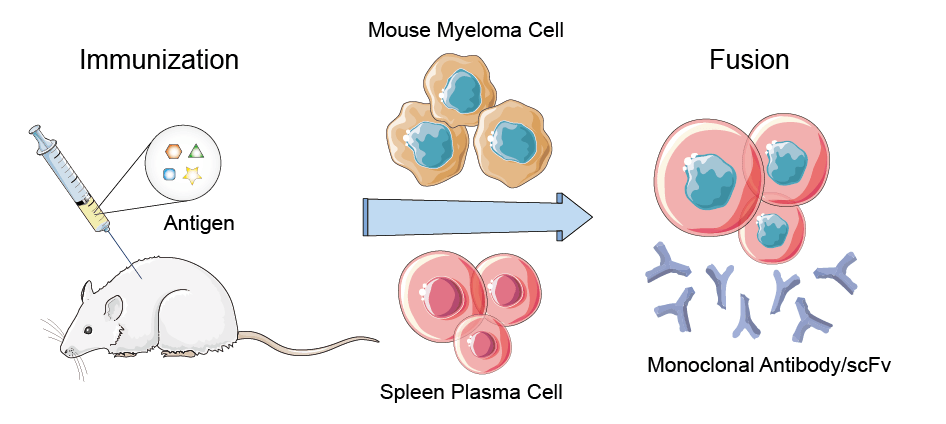 Fig.1 Flowchart of scFv generation from Hybridoma.
Fig.1 Flowchart of scFv generation from Hybridoma.
B. Generate scFv from Phage Display
At Creative Biolabs, the scFv Phage Display is established originally with an immunized phage display scFv library against the interested antigen. Creative Biolabs guarantees the scFv phage library to achieve a capacity of over 1×1010 clones. The detailed procedures are depicted as the following:
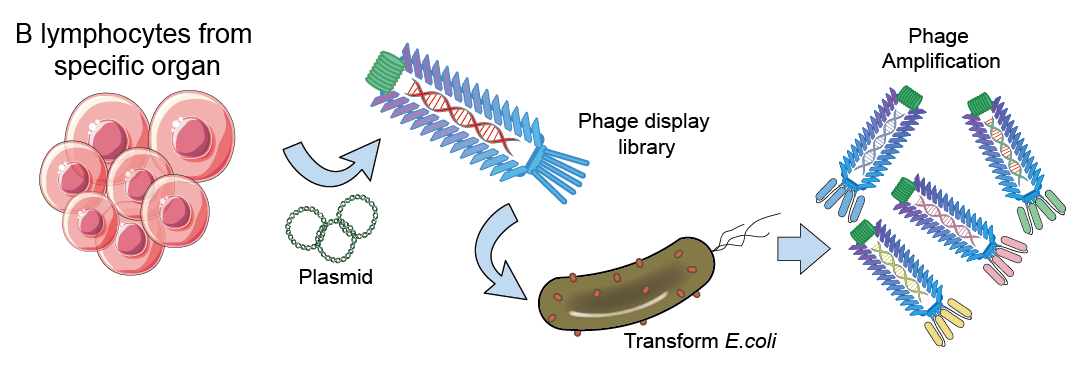 Fig.2 Flowchart of scFv generation from Phage Display.
Fig.2 Flowchart of scFv generation from Phage Display.
In addition, Creative Biolabs also provides optional molecular characterization service during scFv generation, such as western blot, ELISA or other customized requests. Of note, Our world-leading CAR-T library technology make it possible to screen high-affinity and functional CARs based on T cell display and T cell activation. With the aid of this powerful technology, the selected stable clones can be applied in clinic trials immediately. Several CAR-T libraries against CD19, Her2, Her3, EGFR, FGFR1, VEGFR, etc are available to use.
Hemophilia A is a genetic disorder resulting from a deficiency in FVIII, leading to impaired blood clotting. Standard treatment involves FVIII replacement therapy, but many patients develop inhibitory antibodies against the therapeutic protein, complicating treatment. This study aimed to engineer Tregs to suppress the immune response against FVIII, thereby preventing the formation of inhibitory antibodies and enhancing the efficacy of FVIII replacement therapy.
The study employed the following methodology to engineer antigen-specific Tregs:
1. scFv Generation:
2. CAR Treg Engineering:
3. In Vivo and In Vitro Experiments:
The scFvs generated by Creative Biolabs were instrumental in achieving several key outcomes:
1. Specific Targeting:
2. Functional Treg Activation:
3. Immune Response Modulation:
The study provided valuable insights into the differential impacts of CAR and TRuC constructs on Treg function. It demonstrated that while CAR Tregs exhibited robust activation, they lost suppressive functionality and gained an effector phenotype. In contrast, TRuC Tregs, which incorporated the FVIII-specific scFvs into the TCR complex, showed controlled signaling and maintained their suppressive capacity. These findings suggest that TRuC constructs may offer a more effective approach for engineering Tregs to induce immune tolerance in hemophilia A patients. The results highlight the importance of modulating Treg activation thresholds to preserve their suppressive functions and prevent unwanted immune responses.
Creative Biolabs provided crucial scFv generation services, which were foundational to the study. These high-affinity scFvs were designed to specifically target the FVIII protein. The expertise of Creative Biolabs in generating these scFvs enabled the researchers to develop effective CAR constructs for the Tregs.
With professional service and various technology platforms at Creative Biolabs, we offer the best screening and optimization for superior affinity. Meanwhile, the customs sequences of scFv are also welcomed for further optimization, cDNA synthesis, and subsequent service. Our years of experience guarantee we can offer you our high-quality scFv generation. Creative Biolabs offers you world-class scFv construction services to greatly accelerate your research progress with much lower unpredictable risk. Please do not hesitate to contact us.
Fig.3 One-stop CAR-T therapy development process.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION