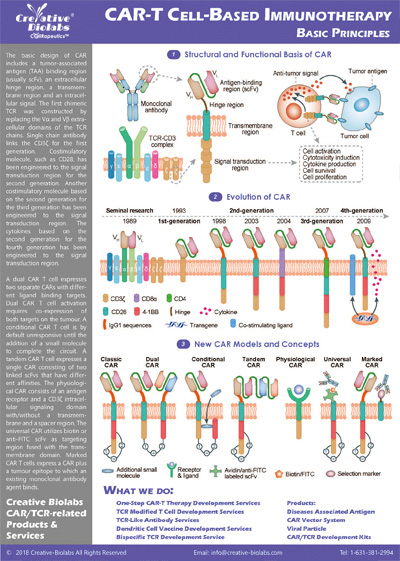
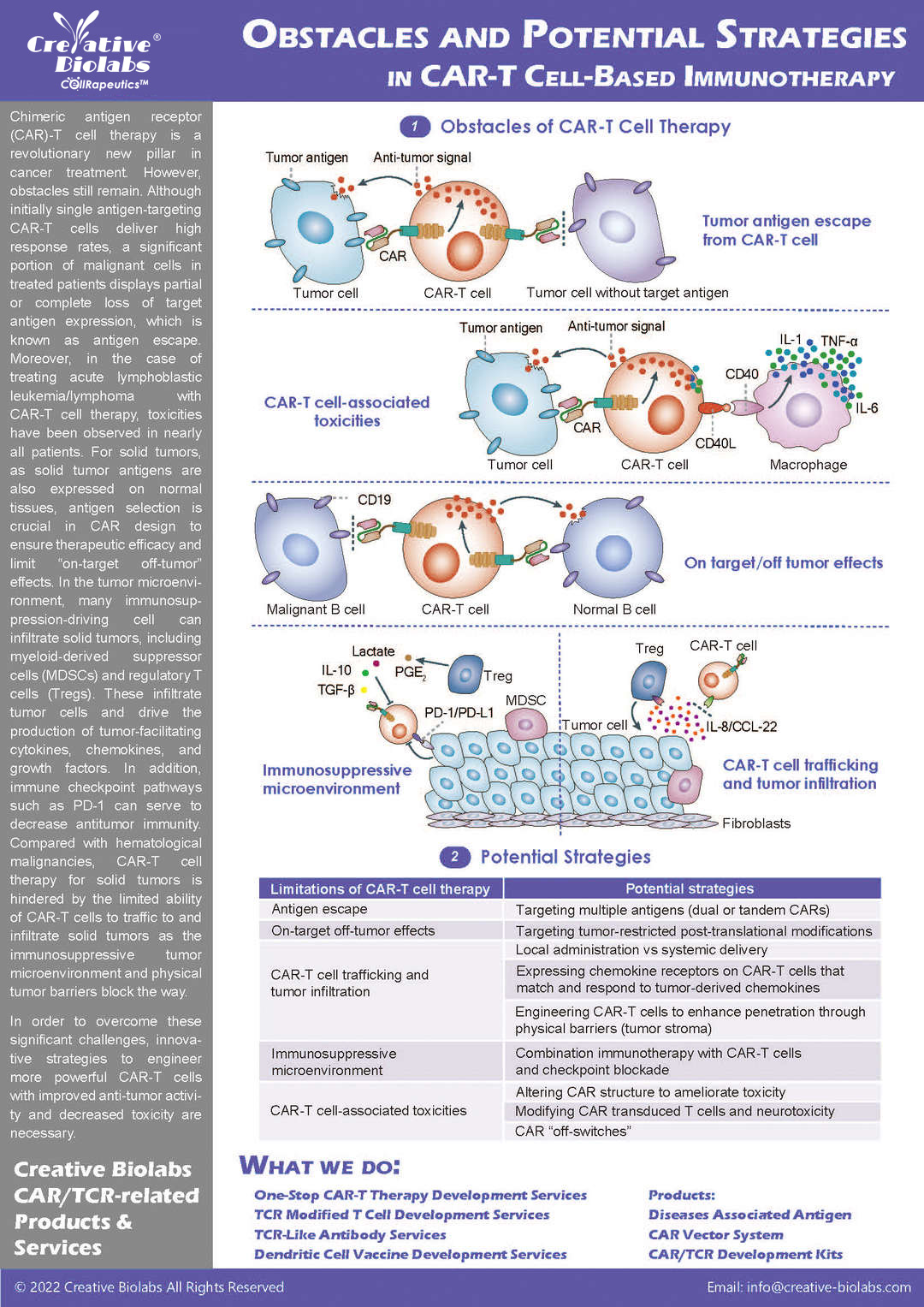
Our CAR design and construction service provides a comprehensive set of deliverables to ensure you have everything you need to move forward with your project. You will receive a fully optimized CAR construct that includes the gene sequence of the entire CAR, designed according to your specifications. We also offer gene sequence optimization to improve expression and reduce potential immunogenicity. Depending on your needs, we can provide the CAR in different vector formats, such as plasmid, lentiviral, or adenoviral vectors, which are fully validated for functionality. Additionally, you will receive detailed reports on sequence verification (e.g., Sanger or next-generation sequencing), expression validation (using methods like flow cytometry or Western blot), and functional testing results, such as cytotoxicity assays. These deliverables give you a fully functional and validated CAR, ready for further in vitro or in vivo studies.

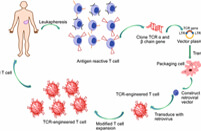
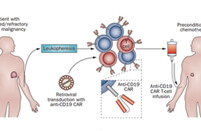






.svg)