All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
The development of allogeneic CAR-T therapy holds great promise for expanding access to innovative treatment approaches and improving outcomes for cancer. Continued research and advancements in this area are essential to realize the full potential of allogeneic CAR-T therapy in the fight against cancer. Creative Biolabs is dedicated to providing unparalleled allogeneic CAR-T development services to foster the next generation of cancer therapy.
Allogeneic CAR-T therapy may be a potential option for patients who are unable to undergo autologous CAR-T therapy due to factors such as poor health, inability to produce enough T cells, or difficulty with T cell collection.
Allogeneic CAR-T therapy can be engineered to target multiple antigens, potentially improving the effectiveness of treatment for certain types of cancer. Additionally, allogeneic CAR-T therapy can be modified to enhance its anti-tumor activity or overcome immune evasion mechanisms utilized by cancer cells.
Our comprehensive allogeneic CAR-T therapy development platform is a multi-faceted approach that encompasses a range of technologies and processes to create a robust and efficient system for engineering and manufacturing CAR-T cells. This platform typically includes the use of gene editing tools, such as CRISPR/Cas9, to modify donor T cells to express the desired CAR targeting a specific antigen on tumor cells. It also involves optimized cell expansion and culture techniques to rapidly generate large quantities of CAR-T cells for clinical use. Quality control measures are implemented throughout the process to ensure the safety and efficacy of the final product. Additionally, our platform includes strategies for managing potential immune reactions and improving the persistence and anti-tumor activity of the CAR-T cells.
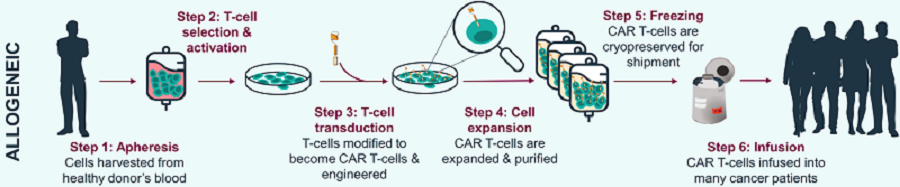 Fig.1 Allogeneic CAR-T manufacturing process from PBMCs.1
Fig.1 Allogeneic CAR-T manufacturing process from PBMCs.1
Allogeneic CAR-T therapy holds promise as a potentially more accessible, cost-effective, and effective treatment option for cancer treatment. Creative Biolabs is exploring the potential of allogeneic CAR-T therapy to address challenges associated with autologous CAR-T therapy and further improve outcomes.
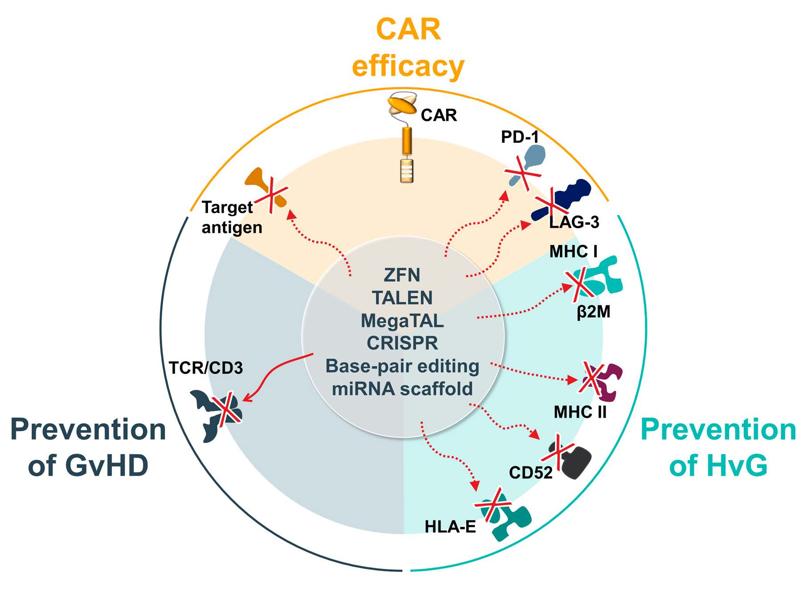 Fig.2 Allogeneic CAR-T therapy technologies.1
Fig.2 Allogeneic CAR-T therapy technologies.1
If you are looking for allogeneic CAR-T therapy development services, contact us today. Our team of experts specializes in developing cutting-edge therapies that utilize allogeneic CAR-T technology. We offer a range of services including preclinical development, process development, and analytical development. Contact us today to learn more about how we can help advance your therapy development goals.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION