In recent years, the field of cancer immunotherapy has seen significant advancements in antibody therapies and cell-based therapies. One novel approach combining antibody and cell therapy has attracted a lot of attention, called STAb immunotherapy, based on endogenous T cells redirecting the secretion of bispecific antibodies (bsAbs). As experts in the field of antibody engineering and immunotherapy, Creative Biolabs continues to lead the way in developing a cutting-edge STAb immunotherapy solution for cancer treatment.
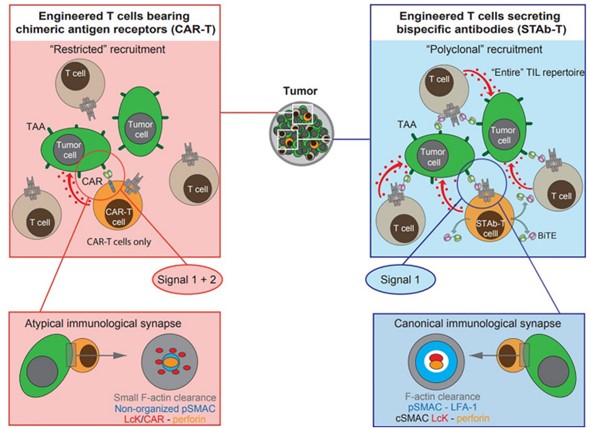 Fig.1 Schematic diagram of CART and STAb strategies.1
Fig.1 Schematic diagram of CART and STAb strategies.1
This innovative strategy combines the benefits of CART cell therapy and systemic infusion of T-bsAbs, providing a promising avenue for cancer immunotherapy. We deliver this solution by engineering T cells or other immune cells to produce different types of antibodies locally (STAb on the tumor) or systemic (STAb outside the tumor) in the tumor microenvironment in vivo, addressing issues such as short serum half-life, tumor penetration, and systemic toxicity. With in-depth knowledge and experience in biologics research and development, Creative Biolabs specializes in providing the following services for global customers, including but not limited to:
Tailored design and construction of STAb vectors for T cell-redirecting strategies.
Precision engineering of vectors to enhance T cell activity towards cancer cells by targeting tumor-associated antigens (TAAs).
Utilization of advanced molecular techniques to ensure successful production of STAb vectors for personalized immunotherapy approaches.
Implementation of in vivo and ex vivo gene-modified strategies to enhance therapeutic efficacy.
Including a variety of in vitro and in vivo experiments, such as immune experiments, animal model selection and in vivo experimental design, in vivo efficacy and pharmacokinetic validation.
Here are some representative data for the STAb strategy application.
This paper introduced a novel strategy using CD1a x CD3 T Cell Engagers (TCEs) specifically designed for CD1a+ T-ALL patients. The engineered T cells expressing soluble CD1a x CD3 TCEs successfully bind to CD1a and CD3 on T-ALL blasts, leading to targeted T cell activation. In comparison to CD1a-CAR T cells, CD1a x CD3 TCE-transduced T cells exhibit different subpopulations and demonstrate the ability to activate bystander T cells, showing enhanced cytotoxic responses. The use of CD1a-STAb T cells triggers a potent and rapid cytotoxic response with a proinflammatory cytokine profile, using granular exocytosis as a common mechanism of action. Moreover, the bystander recruitment ability of CD1a-STAb T cells provides efficient tumor cell killing, potentially offering a safer therapeutic profile than conventional CAR T cells. These findings suggest that CD1a-STAb T cells could provide an alternative and effective treatment for co-T-ALL patients, delivering equal or superior cytotoxicity capacity without exaggerated cytokine release-associated side effects.
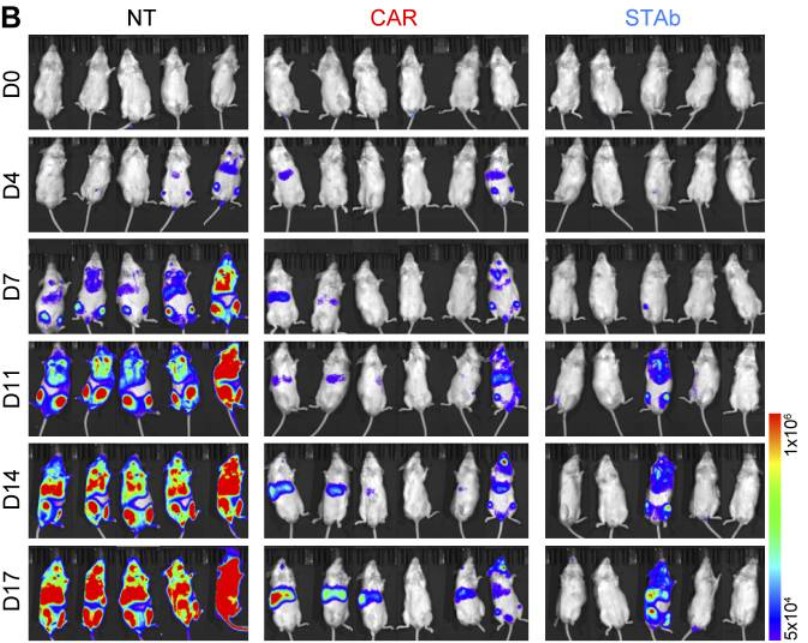 Fig.2 In vivo efficacy assessment of CD1a-STAb T cells in the T-ALL model.2
Fig.2 In vivo efficacy assessment of CD1a-STAb T cells in the T-ALL model.2
Q: What are the potential advantages of STAb solution comparing conventional immunotherapy solutions?
A: In comparing other strategy, STAb solution possesses many potential advantages, such as effective and persistent antibody concentrations, bypassing concerns over storage and formulation, and the ability to redirect non-engineered T cells to target tumor cells.
With a wealth of experience, expertise, and a commitment to excellence, Creative Biolabs is at the forefront of revolutionizing cancer immunotherapy through novel approaches. We focus on customized services to meet the unique needs and requirements of each client, ensuring the best results in the customers' projects. Please feel free to get in touch with us.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION