All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Studies have shown that hematopoietic stem cells (HSCs) play a crucial role in the development of chimeric antigen receptor (CAR) T-cell therapies. CAR-T cells, on the other hand, are engineered T cells that are capable of recognizing and attacking cancer cells. HSCs are responsible for producing all types of blood cells, including T cells, which are the basis of CAR-T cell therapy.
Creative Biolabs has launched the allogeneic hematopoietic stem cell CAR-T development service which includes various stages of the CAR-T therapy development process, such as cell line engineering, optimization of gene delivery methods, and preclinical evaluation of safety and efficacy. Expertise in cell biology, immunology, and gene editing technologies, please feel free to entrust your project to us.
Creative Biolabs' allogeneic hematopoietic stem cell platform involves obtaining hematopoietic stem cells from healthy donors and genetically modifying them to express CAR. One of the main advantages of using allogeneic hematopoietic stem cells for CAR-T therapy is that it allows the creation of off-the-shelf CAR-T products that can be easily obtained without the need for individualized cell production. This has the potential to reduce costs and improve access to CAR-T therapies.
Expertise in hematopoietic stem cell
One of the key aspects of our service is the application of genome engineering to hematopoietic stem cells (HSCs). By leveraging cutting-edge technologies, we are able to modify and enhance the functionality of HSCs, opening up new possibilities for personalized medicine and targeted therapies.
Applying genome engineering to HSCs
Creative Biolabs has a deep understanding of the molecular mechanisms involved in HSC biology and have successfully utilized cutting-edge tools such as CRISPR/Cas9 technology to manipulate the genomes of these cells. Our expertise in this area has enabled us to create genetically modified HSCs with precise alterations, allowing for more targeted research and therapeutic applications.
Unleash the potential of targeted therapy productions
Through our allogeneic hematopoietic stem cell development service, we aim to unleash the full potential of targeted therapies by optimizing HSCs for specific treatments. This tailored approach allows for more precise, effective, and personalized treatments for a wide range of diseases and conditions.
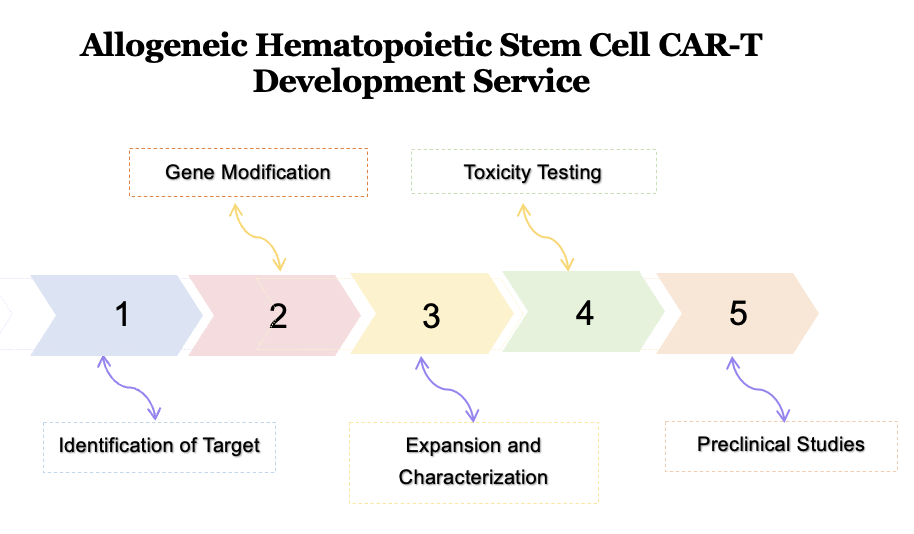
The first step in the development process of Allogeneic Hematopoietic Stem Cell CAR-T therapy is the identification of a suitable target antigen for the therapy. This antigen should be highly expressed on cancer cells but minimally present on normal cells to minimize off-target effects.
The second step is to genetically modify allogeneic hematopoietic stem cells to express CARs that target the identified antigens. This involves the insertion of the CAR gene into the stem cells using viral vectors or other gene delivery methods.
After genetic modification, the cells are expanded. During this process, the cells are characterized to ensure that they express the CAR and have the desired properties for the therapy.
Before moving on to clinical trials, the modified stem cells undergo rigorous testing to assess their safety and potential toxicity. Creative biolabs provides in vitro and in vivo studies to evaluate potential off-target effects and immune responses.
Once the safety and efficacy of the modified stem cells have been established, preclinical studies are conducted to evaluate the therapeutic potential of the therapy in animal models of cancer.
Allogeneic hematopoietic stem cell CAR-T therapy has shown promising results in the treatment of various types of cancer, including leukemia, lymphoma, and solid tumors. By genetically modifying these stem cells to express chimeric antigen receptors (CARs), they can specifically target and destroy cancer cells while sparing healthy tissues.
Allogeneic hematopoietic stem cell CAR-T therapy has the potential to be used in the treatment of autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. By reprogramming the stem cells to regulate the immune response, they can help restore immune tolerance and prevent the progression of these diseases.
Allogeneic hematopoietic stem cell CAR-T therapy can also be utilized in regenerative medicine to repair and regenerate damaged tissues and organs. By directing these stem cells to differentiate into specific cell types, they can help in the repair and regeneration of tissues damaged by injury, disease, or aging.
In addition, allogeneic hematopoietic stem cell therapy can be used to develop new therapies against infectious diseases such as HIV, hepatitis B and C, and tuberculosis. By engineering the stem cells to express receptors that target pathogens, they can be used to eliminate infected cells and enhance the immune response against pathogens.
If you are interested in learning more about our Allogeneic Hematopoietic Stem Cell Development Service, please do not hesitate to contact us. Our team of stem cell experts is committed to providing you with the latest information and technical support on this cutting-edge technology. We're always here around the clock to address any questions or concerns you may have and help you get the most out of this service. Reach out to us now to start the conversation about how Allogeneic Hematopoietic Stem Cell Development can benefit you and your research objectives.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION