All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Allogeneic NK cell therapy shows promise in the treatment of certain types of cancer, such as leukemia, lymphoma, and solid tumors. NK cells can be derived from a variety of sources for therapeutic use, including peripheral blood, umbilical cord blood (UCB), and induced pluripotent stem cells. Among the various sources for NK cells, UCB-derived NK cells present distinct advantages, including their availability, stronger proliferation potential, less mature, ease of collection expansion and storage, and lower risk of graft-versus-host disease (GVHD). Therefore, allogeneic UCB-derived NK cells hold great promise for the development of effective and safe cell-based therapies for various diseases, particularly in the field of cancer immunotherapy.
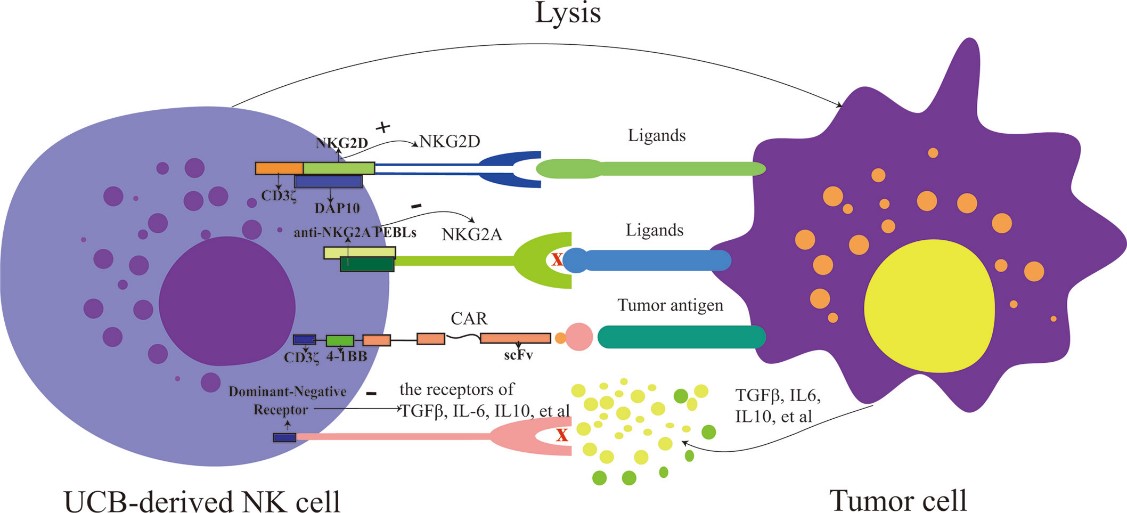 Fig.1 Modified UCB-derived NK cells.1
Fig.1 Modified UCB-derived NK cells.1
Creative Biolabs is a leading provider of UCB-derived NK cell development services, bringing state-of-the-art solutions to researchers in the field of immunotherapy. Our team of experts specializes in isolating, culturing, expanding, and modifying UCB-derived NK cells for use in cancer therapy. With our cutting-edge technology and extensive experience in the field, we can customize NK cells to specifically target and destroy cancer cells.
In the commitment to advancing immunotherapy, Creative Biolabs has established a robust allogeneic UCB-derived NK dell platform aimed at delivering customized and high-quality NK cell products. Creative Biolabs combines innovative techniques with decades of expertise in immunology and cellular biology to provide tailored solutions that meet specific research and clinical requirements. The platform begins with the isolation of UCB-derived NK cells, ensuring high viability and purity. These cells are then expanded using proprietary culture conditions that maximize their cytotoxic potential and functional longevity. The UCB-derived NK cells generated through our protocol exhibit consistently high expression levels of activating NKG2D and natural cytotoxicity receptors, maximizing their anti-cancer efficacy.
Our platform also encompasses genetic modifications to enhance the efficacy and safety of NK cells. By using cutting-edge gene-editing technologies, UCB-derived NK cells can be further engineered with specific genetic modifications, such as chimeric antigen receptors (CARs), to optimize NK cell function, enhance their tumor-targeting capabilities, and minimize the risk of off-target effects. This comprehensive approach ensures that NK cells are not only potent but also tailored to clients' unique study requirements. Additionally, we offer extensive preclinical testing services, providing crucial insights into the therapeutic potential and safety profile of UCB-derived NK cells before transitioning to clinical trials.
Creative Biolabs is committed to revolutionizing cancer treatment through the innovative utilization of UCB-derived NK cells. Our UCB-derived NK cell development service is designed to meet the specific needs of each project, providing a tailored and innovative approach to cancer immunotherapy. From the initial consultation to the final delivery of your NK cell products, Creative Biolabs ensures a collaborative and productive partnership to facilitate groundbreaking discoveries and therapeutic advancements. If you are interested in developing the potential of UCB-derived NK cells for cancer treatment, contact us today to learn more about how we can help you with your project.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION