T cell deficiencies significantly impair the immune system, making patients highly susceptible to various infections and diseases. Current treatments for T cell deficiencies aim to boost the immune system and manage infections. These include antiviral drugs like ganciclovir for CMV, antibiotics for bacterial infections, antifungal medications such as fluconazole, and immunoglobulin replacement therapy through regular intravenous or subcutaneous injections. Hematopoietic stem cell transplantation (HSCT) is used in severe cases to rebuild the immune system with healthy donor stem cells. Gene therapy addresses specific congenital T cell deficiencies by repairing genetic defects. Current treatments for T cell deficiencies, while varied and essential, often fall short of providing comprehensive immune support. The development of advanced virus-specific T cell (VST) therapies promises superior outcomes by directly addressing immune deficits and offering targeted viral protection.
At Creative Biolabs, we lead the forefront in VST therapy development. Our specialized services harness cutting-edge techniques, including custom VST design, optimized antigen selection, and high-efficiency T cell expansion.
Personalized VST Development: We customize VSTs by stimulating PBMCs with virus peptide libraries representing viral antigens of interest.
Polyclonal T Cell Production: Our process yields large numbers of polyclonal CD4+ and CD8+ T cells capable of targeting multiple viruses simultaneously.
Comprehensive Phenotypic Analysis: We conduct detailed phenotypic profiling to characterize the surface markers and functional attributes of VSTs.
Functional Assays: Rigorous functional assays validate the antiviral potency of VSTs against specific viral targets, ensuring therapeutic efficacy.
Comprehensive Antigen Screening: Extensive screening identifies immunogenic viral epitopes crucial for enhancing VST specificity.
Efficient Antigen Loading: Proprietary methods optimize antigen presentation, enhancing VST effectiveness in combating targeted viruses.
Scalable Expansion Protocols: Protocols designed for scalable production of VSTs ensure sufficient quantities.
Quality Control Measures: Stringent quality control guarantees the consistency and purity of VST batches.
Advanced Bioreactor Technology: Utilization of advanced bioreactor systems enhances VST yield and quality.
Advanced Cryopreservation Techniques: Cutting-edge methods preserve VST viability and functionality for extended storage periods.
Secure Storage Facilities: State-of-the-art infrastructure maintains optimal conditions.
This article explores the application of adoptive virus-specific T-cell therapy in treating infections affecting the CNS. It discusses how this therapy leverages the immune system's ability to target and eliminate viral pathogens that invade the CNS, such as herpesviruses and other neurotropic viruses. The review emphasizes the therapeutic potential of virus-specific T cells, which are engineered or selected to recognize and attack infected cells while sparing healthy tissues. It also highlights clinical outcomes and challenges associated with implementing this therapy, including the need for specialized protocols to ensure efficacy and safety in treating severe CNS infections. Overall, the article underscores the promising role of virus-specific T-cell therapy in addressing challenging viral infections of the central nervous system.
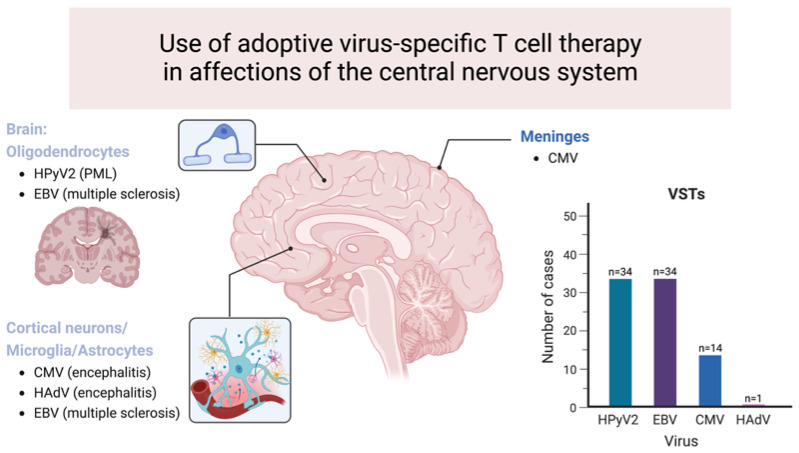 Fig.2 Use of adoptive VST-cell therapy in affections of the CNS.1
Fig.2 Use of adoptive VST-cell therapy in affections of the CNS.1
Q1. How do VSTs work?
A1: VSTs are specialized immune cells that recognize and target specific viral infections. They are derived from healthy donor cells and can be tailored to combat multiple viruses simultaneously.
Q2. What types of viruses can VST therapy target?
A2: VST therapy can target a wide range of viruses, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), and others that pose risks to immunocompromised patients.
Q3. How effective is VST therapy compared to traditional treatments?
A3: VST therapy offers targeted immune support specifically tailored to combat viral infections, potentially providing more effective and sustained protection compared to traditional antiviral drugs and other treatments.
Connect with us today to discover how our advanced VST therapy development services can meet your specific needs. Partner with Creative Biolabs for innovative solutions in enhancing immune response and combating viral diseases effectively.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

