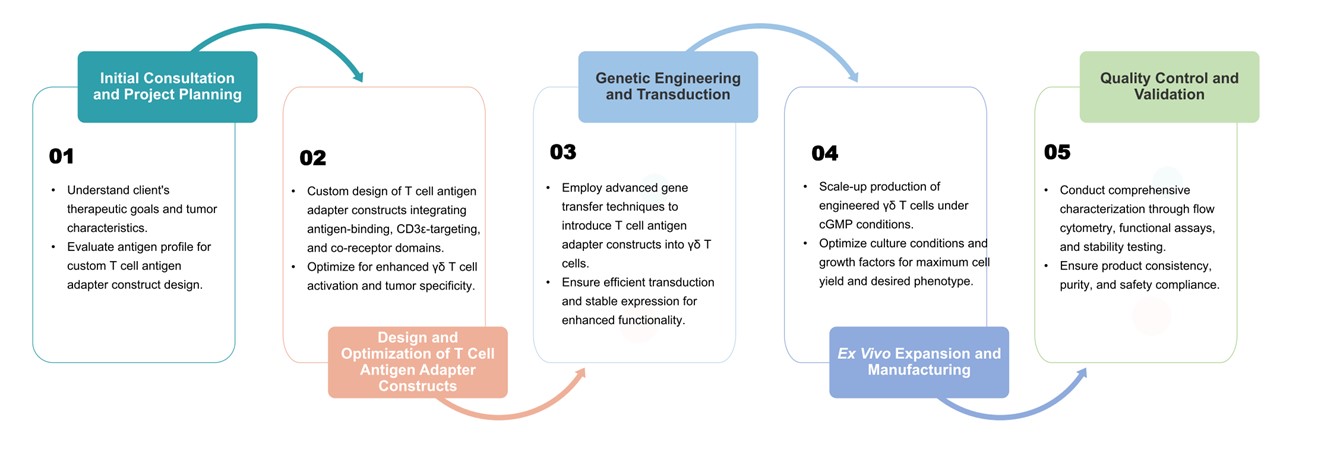
Creative Biolabs proudly introduces our Allogeneic γδ T Cell Development Service, leveraging cutting-edge T cell antigen adapter technology. Our T cell antigen adapter is composed of an antigen-binding domain, a cd3ε-targeting domain, and a co-receptor. We specialize in engineering γδ T cells to enhance their tumor-targeting capabilities, offering potent and personalized solutions in cancer immunotherapy.
Advantages of γδ T Cells:
Antigen-binding Domain Selection: We tailor the design of the T cell antigen adapter construct to target specific tumor antigens identified through comprehensive tumor profiling.
CD3ε-targeting Domain Incorporation: Integration of CD3ε-targeting domains ensures robust T cell activation upon antigen recognition, enhancing cytotoxic response against cancer cells.
Co-receptor Optimization: Optimization of co-receptor components to enhance signal transduction pathways and promote sustained T cell activity within the tumor microenvironment.
Utilization of cutting-edge gene transfer technologies to introduce engineered T cell antigen adapter constructs into γδ T cells.
Optimization of transduction efficiency and stability to achieve high levels of construct expression and functional activity in γδ T cells.
Scalable production of engineered γδ T cells to ensure safety, potency, and consistency.
Expansion protocols optimized for maximum cell yield while maintaining desired phenotype and functional properties necessary for therapeutic efficacy.
Comprehensive characterization of engineered γδ T cells through rigorous quality control assays, including flow cytometry, cytotoxicity assays, cytokine production assays, and stability testing.
At Creative Biolabs, we are dedicated to pioneering innovative solutions in cancer immunotherapy. Our allogeneic γδ T cell development service leverages advanced T cell antigen adapter technology to provide enhanced, scalable, and personalized therapeutic options. If you are looking to harness the power of engineered γδ T cells for your cancer treatment strategies, we invite you to contact us. Our team of experts is ready to collaborate with you to develop cutting-edge, effective therapies tailored to your needs.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION

