All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Chronic hepatitis B virus (HBV) can cause decreased immunity and lead to the development of liver cancer. Based on CAR-T cell therapy, Creative Biolabs has designed a CAR-T cell that specifically recognizes Hepatitis B virus surface antigen (HBsAg) and provides in vitro/in vivo evaluation services.
Existing treatments, such as antiviral medications (e.g., tenofovir, entecavir), can suppress HBV replication but do not typically eliminate the virus. These treatments often need to be taken lifelong and do not offer a cure. Anti-HBV CAR cell therapy is an advanced immunotherapy approach designed to treat chronic HBV infection. This therapy involves genetically modifying immune cells to specifically target and eliminate HBV-infected cells.
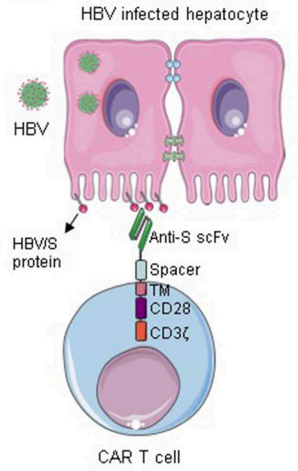 Fig.1 CAR T cells specific for HBV S protein.1
Fig.1 CAR T cells specific for HBV S protein.1
Creative Biolabs provides a one-stop solution for anti-HBV CAR cell therapy, a cutting-edge approach to the treatment of HBV infection. Here is a detailed description of each step:
Creative Biolabs identifies suitable antigens present on the surface of HBV-infected cells. This antigen must be specific to the infected cells to ensure that CAR T cells selectively target and destroy only these cells.
Creative Biolabs designed and constructed a CAR that recognizes identified HBV antigens.
Creative Biolabs isolated T cells that have been genetically modified to express CARs that are specific for HBV antigens.
Creative Biolabs amplified CAR-expressing genetically engineered T cells to generate a sufficient number of cells for treatment.
As a leading supplier in the field of CAR, we also offer in vitro and in vivo evaluation services for HBV CAR:
Anti-HBV CAR cell therapy development has several important applications, primarily focused on treating chronic HBV infections and related complications. Here are some key applications:
Anti-HBV CAR cell therapy is being pursued because it offers a promising new approach to potentially cure chronic HBV infection, overcome the limitations of current treatments. If you are interested in this Anti-HBV CAR Cell Therapy Development Solutions, please feel free to contact us. Our technicians in the relevant field will be happy to assist you.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION