All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs offers a comprehensive range of services for the development of anti-HCV CAR T-cell therapies. With expertise in molecular biology, immunology, virology and cell therapy, we ensure a high-quality R&D process for you.
While direct-acting antivirals (DAAs) have revolutionized the treatment of HCV, they are not applicable to all ranges and are not always curative. Some patients fail to achieve a sustained virologic response (SVR) and may experience relapse. Anti-HBV CAR cell therapy represents a promising and innovative approach to treating chronic HBV infection. By harnessing the power of genetically engineered T cells to specifically target and eliminate HBV-infected cells, this therapy aims to provide a functional cure and overcome the limitations of current antiviral treatments.
 Fig.1 CAR T cells specific for HCV/E2.1
Fig.1 CAR T cells specific for HCV/E2.1
Creative Biolabs provides anti-HCV CAR cell therapy development services aimed at targeting and eliminating HCV-infected cells. This service includes a range of activities, from the initial design of CAR constructs to preclinical testing of in vitro and in vivo models.
Creative Biolabs provides Biomarker Identification & Selection service for the identification of specific HCV antigens that can be targeted by CAR T cells. HCV CAR Design: CAR constructs were designed to include antigen recognition domains, transmembrane domains, and intracellular signal transduction domains.
Creative Biolabs provides genetic modification services for T cells, including: T Cell Isolation; Gene Transfer; Expansion.
Creative Biolabs provides HCV CAR in vitro testing services including, but not limited to: assessing the ability of CAR T cells to kill HCV-infected cells using assays such as lactate dehydrogenase (LDH) release or flow cytometry-based assays; Measure cytokines (e.g., IFN-γ, TNF-α) produced by CAR T cells when they encounter HCV-infected cells; To assess the proliferation of HCV antigens by CAR T cells.
Creative Biolabs provides HCV CAR in vivo testing services, including but not limited to: Immunodeficient mouse models of transplantable human hepatocytes or HCV-infected cells were used to evaluate the efficacy and safety of CAR T cells in organisms; monitor the reduction of viral load and clearance of HCV-infected cells in animal models; Track the distribution and persistence of CAR T cells in different tissues; To evaluate the potential off-target effects and overall safety of CAR T cells.
Creative Biolabs helps you improve the CAR structure to improve its specificity, potency, and durability, and conduct rigorous testing to verify the effectiveness and safety of the optimized CAR T cells.
Here we present the important results of a study that designed CAR-T cells against the HCV E2 glycoprotein (HCV/E2). These findings will help you a better understanding of our anti-HCV CAR cell therapy development services.
| CAR Construction | |
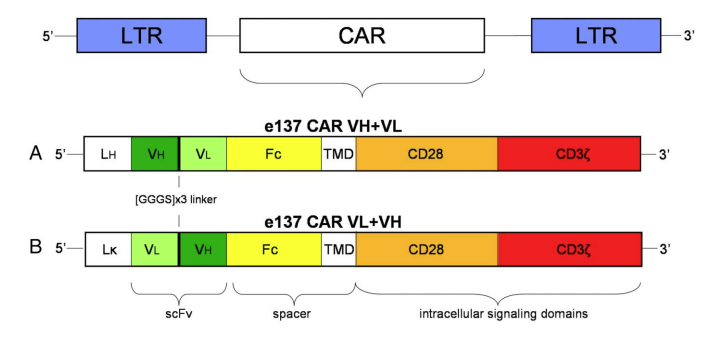 Fig.2 CAR construction against HCV E2 glycoprotein.2 |
The figure on the left shows a schematic representation of the engineered e137-CAR. |
| Cytokine Release Test | |
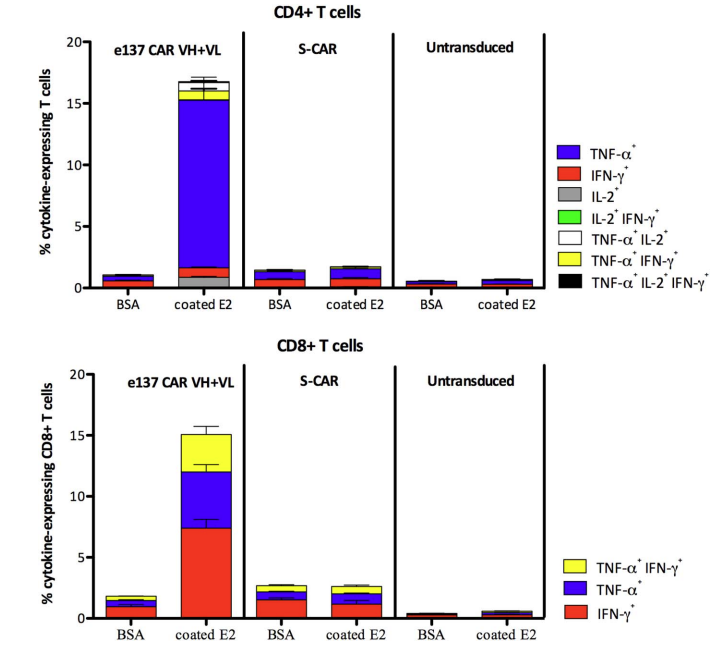 Fig.3 Cytokine assay for e137-CAR engineered T cells.2 |
The left figure assesses the activation of T cells redirected by e137-CARs by detecting secreted cytokine levels. |
| In Vitro Cytotoxicity Test | |
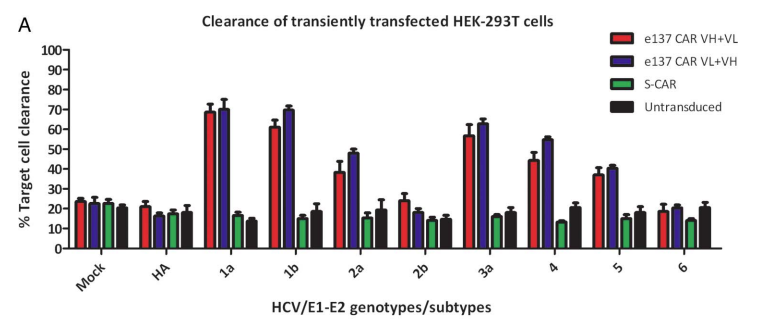 Fig.4 Cytotoxicity test for e137-CAR engineered T cells.2 |
The left figure shows the results of the cytotoxic activity of e137-CAR-redirected engineered T cells against different HEK-293T target cells. |
The development of anti-HCV CAR cell therapy represents a significant advancement in the treatment of chronic HCV infection. By providing targeted, effective, and potentially curative treatment options, CAR T cell therapy addresses the limitations of current treatments:
Creative Biolabs addresses the limitations of current anti-HCV therapies by utilizing advanced immunotherapy technologies and continues to explore the broader field of viral immunotherapy. You can click here to learn more about CART development services for infectious diseases, or get in touch with one of our technicians directly for more information you need.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION