All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
T cells, a subset of lymphocytes, derive from hematopoietic precursor cells in the bone marrow and undergo maturation within the thymus gland. They play an integral role in adaptive immunity, orchestrating targeted immune responses against pathogens and abnormal cells.
Fundamental to immune homeostasis, T cells defend the organism against infectious agents, malignancies, and other pathological conditions. Dysfunctions or imbalances within T cell populations may precipitate immune pathologies, autoimmune disorders, and heightened vulnerability to infections. Thus, a comprehensive comprehension of T cell biology is imperative for advancing therapeutic strategies across diverse pathological contexts.
Immune checkpoint inhibitors
Immune checkpoints are proteins that act as "brakes" on the immune system, preventing excessive immune responses. By blocking these checkpoints, monoclonal antibodies can enhance the activity of T cells and unleash their anti-tumor or anti-pathogen effects. Examples of immune checkpoint inhibitors include antibodies targeting programmed cell death protein 1 (PD-1), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4).
T cell depletion
In certain conditions, such as disorder diseases or organ transplantation, it may be desirable to suppress T cell activity. Monoclonal antibodies selectively deplete or suppress T cells, targeting specific T cell subsets or interfering with their activation and function. For instance, antibodies targeting CD3 are used for T cell depletion.
Chemokine receptors
Chemokine receptors are cell surface receptors that bind to chemokines, small signaling proteins. Chemokine receptors are primarily expressed on the surface of immune cells, including leukocytes such as T cells, B cells, neutrophils, monocytes, and dendritic cells. Monoclonal antibodies can target chemokine receptors like CCR4 (CC Chemokine Receptor 4) on T cells, regulating their trafficking and migration to specific tissues or sites of inflammation.
Programmed Cell Death Protein 1 (PD-1) and CTLA-4 (Cytotoxic T-lymphocyte-associated Protein 4) are immune checkpoint receptors expressed on the surface of T cells. Overexpression can lead to decreased anti-tumor immune response, leading to immune escape or tumor development. Targeting PD-1 and CTLA-4 activates T cells, exerting anti-tumor effects and ultimately resulting in tumor elimination. Current drugs targeting PD-1 include pembrolizumab and nivolumab, utilized in the treatment of various cancers. Ipilimumab, a drug targeting CTLA-4, is employed in melanoma treatment.
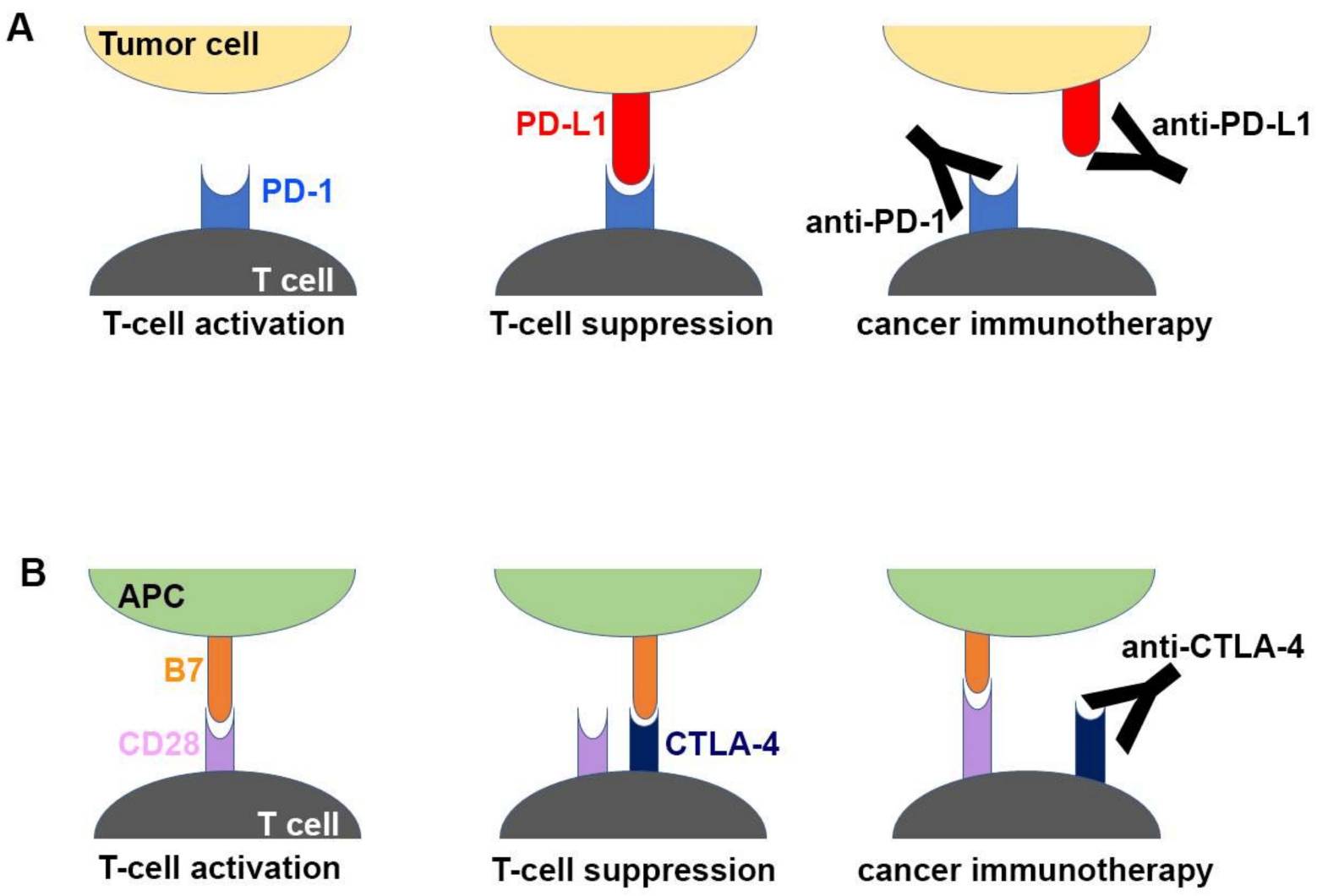 Fig.1. Molecular Mechanism of Checkpoint Blockade by Antibodies (Yong-Seok Heo, 2019)
Fig.1. Molecular Mechanism of Checkpoint Blockade by Antibodies (Yong-Seok Heo, 2019)
CD3 is a complex of proteins expressed on the surface of T cells, comprising four distinct proteins: CD3γ, CD3δ, CD3ε, and CD3ζ. These proteins form heterodimers and heterotrimers, assembling into the CD3 complex that associates with TCRαβ or TCRγδ heterodimers to form the complete TCR complex. It plays a crucial role in T cell signaling and activation. When the TCR binds to its specific antigen presented by major histocompatibility complex (MHC) molecules on antigen-presenting cells (APCs), the CD3 complex transduces signals into the T cell, leading to downstream signaling events that ultimately activate the T cell. This activation is essential for the T cell to carry out its effector functions, such as cytokine production, proliferation, and cytotoxicity. Dysregulation of CD3-mediated signaling can lead to immune disorders, such as immunodeficiency or autoimmune diseases.
Monoclonal antibodies targeting CD3 offer a targeted approach for modulating immune responses in autoimmune disorders and organ transplantation. They selectively deplete activated T cells, providing precision in immunomodulation.
Muromonab-CD3, a pioneering murine-derived monoclonal antibody, acts as a potent immunosuppressive agent, particularly in kidney transplant recipients, by binding to the CD3 complex on T cells. Its FDA approval in 1986 marked a milestone in transplantation medicine, showcasing the potential of monoclonal antibody-based immunosuppression.
Otelixizumab, a humanized monoclonal antibody targeting CD3, has demonstrated promise in attenuating the autoimmune response in conditions such as type 1 diabetes and rheumatoid arthritis.
Teplizumab, another humanized monoclonal antibody against CD3, aims to induce immune tolerance and preserve pancreatic beta-cell function in recent-onset type 1 diabetes.
Visilizumab, also humanized, is under investigation for preventing organ rejection, especially in kidney transplantation, by inhibiting T cell activation and proliferation.
Collectively, these monoclonal antibodies underscore the diverse therapeutic potential of CD3-targeted therapies, ranging from immunosuppression in transplantation to immune modulation in autoimmune diseases.
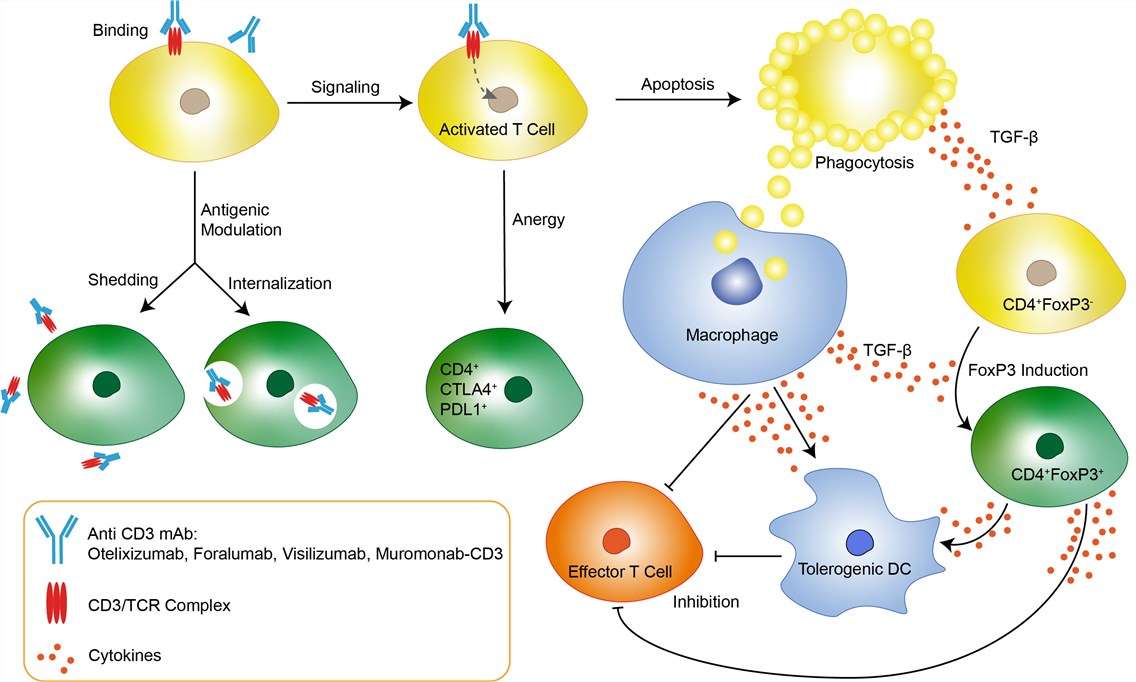 Fig.2. Mechanism of Action of Muromonab-CD3 (Created Biolabs)
Fig.2. Mechanism of Action of Muromonab-CD3 (Created Biolabs)
CCR4 (C-C Chemokine Receptor Type 4) belongs to the family of chemokine receptors, which are cell surface receptors that bind to chemokines, and is expressed on certain types of T cells (e.g., Th2 cells, regulatory T cells) and dendritic cells. CCR4 plays a critical role in regulating immune responses by mediating the migration and homing of CCR4-expressing cells to specific tissues and sites within the body. It directs T cell trafficking to sites of inflammation, where they exert their effector functions.
Aberrant expression or activation of CCR4 has been implicated in various immune-mediated diseases and conditions, including allergic diseases (such as asthma and allergic rhinitis), autoimmune diseases (such as rheumatoid arthritis and multiple sclerosis), and certain types of cancer (such as cutaneous T-cell lymphoma and peripheral T-cell lymphoma). In cancer, CCR4 is often overexpressed on malignant T cell' surface, making it an attractive therapeutic target.
Monoclonal antibodies targeting CCR4 exert anti-cancer effects through antibody-dependent cellular cytotoxicity (ADCC), antibody-dependent cellular phagocytosis (ADCP), and complement-dependent cytotoxicity (CDC). These patterns lead to cancerous T cell destruction and tumor growth inhibition. The drug Mogamulizumab is approved for treating certain types of T cell lymphomas and peripheral T cell lymphomas.
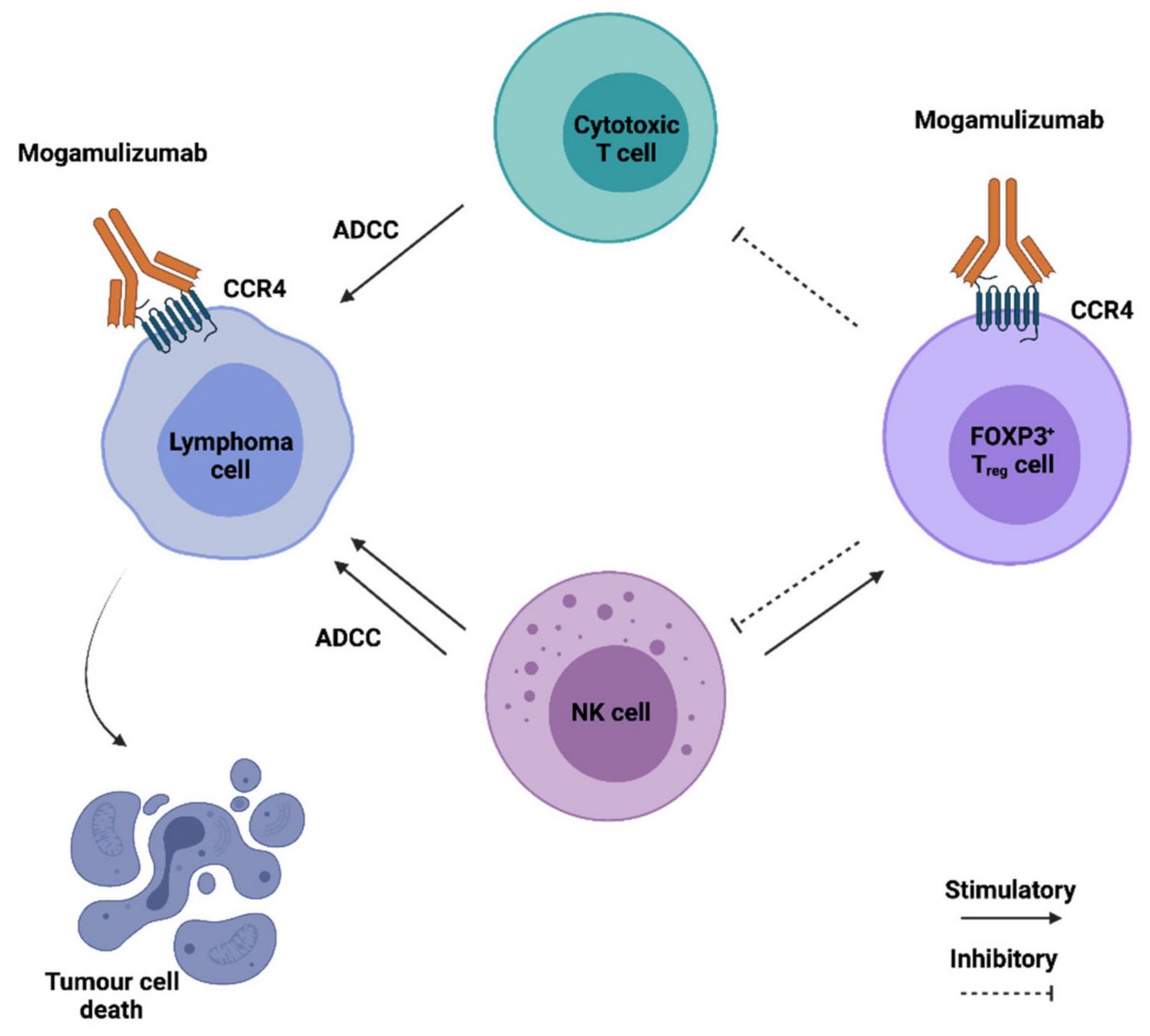 Fig.3. Mechanism of Action of Mogamulizumab (Joana Nunes Ribeiro Dias, 2021)
Fig.3. Mechanism of Action of Mogamulizumab (Joana Nunes Ribeiro Dias, 2021)
Empowered by our state-of-the-art CellRapeutics™ Chimeric Antigen Receptor (CAR) Technology, Creative Biolabs offers world-leading CAR-T therapy development services, aiming to improve the ability of CAR cells to attack solid tumors. Our one-stop services covering Biomarker Identification & Selection, ScFv Generation, CAR Design & Construction, Virus Packaging & CAR Gene Delivery, CAR-T In Vitro Assay, CAR-T Preclinical In Vivo Assay, and CAR-T Clinical Trial. We also provide CAR-T Vector Products, CAR Cell Products, and a proprietary online system for customizing CAR and CAR cell products, which offers full options to meet all unique needs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION