All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
The advancement of cancer immunotherapy has highlighted the need for effective and predictive preclinical models. Among the most promising developments is the use of Peripheral Blood Mononuclear Cell (PBMC) humanized mice models. These models are particularly useful for studying the human immune system's response to cancer vaccines, aiding in the design of personalized treatment strategies. Creative Biolabs has leveraged its extensive expertise to enhance these models for various cancer applications.
PBMC humanized mice have shown exceptional utility in the field of immuno-oncology. By integrating patient-derived xenografts (PDX) and human immune cells, these models enable the study of immune-tumor interactions in a near-physiological context.
Given their ability to reflect individual patient immune responses, PBMC humanized models are increasingly being utilized in personalized medicine. The incorporation of autologous immune cells allows for the assessment of patient-specific tumor responses, thereby providing a predictive tool for clinical outcomes.
The PBMC humanized mouse model is invaluable in developing cancer vaccines. By enabling the study of human immune responses, these models facilitate the identification and validation of tumor-specific antigens. This is particularly crucial for developing vaccines targeting neoantigens, which are pivotal for eliciting robust and specific anti-tumor immunity.
Creative Biolabs offers a state-of-the-art PBMC humanized model specifically designed for cancer vaccine research. This model combines high-quality PDX with autologous or allogeneic PBMCs, enabling the study of human-specific immune responses in in vivo setting. Below are the key features of our model.
Our PBMC humanized model ensures rapid engraftment of human immune cells, typically within 7-14 days post-PBMC injection. This prompt engraftment is validated through flow cytometry analysis for human CD45+ cells, indicating successful reconstitution of T and B cells.
Creative Biolabs provides a highly flexible platform for customizing study designs based on research requirements. Whether it's for short-term proof-of-concept studies or more extended analyses, our model can be tailored to address specific scientific questions effectively.
Cost-Effectiveness
Compared to CD34+ HSC humanized models, our PBMC-based models offer a more cost-effective solution. The simplified engraftment process and rapid setup minimize both time and financial investment, making it a viable option for various research endeavors.
Versatile Applications
Our model supports a wide range of applications, from studying checkpoint inhibitors to evaluating the efficacy of novel cancer vaccines. It is suitable for various tumor types, including breast, colon, lung, and melanoma, providing a comprehensive tool for cancer research.
Minimized Graft versus Host Disease (GvHD)
The employment of specific mouse strains with genetic modifications (e.g., NSG-dKO) helps mitigate GvHD, extending the study window and improving the interpretability of long-term efficacy and toxicity studies.
Multiple studies have validated the utility of PBMC humanized models for cancer vaccine research. For instance, autologous humanized mice have demonstrated higher concordance with patient responses when treated with anti-PD-1 therapies. This correlation underscores the model's capacity to engage pre-existing tumor-specific T-cells, making it a valuable tool for evaluating immune checkpoint inhibitors and other immunotherapeutic agents.
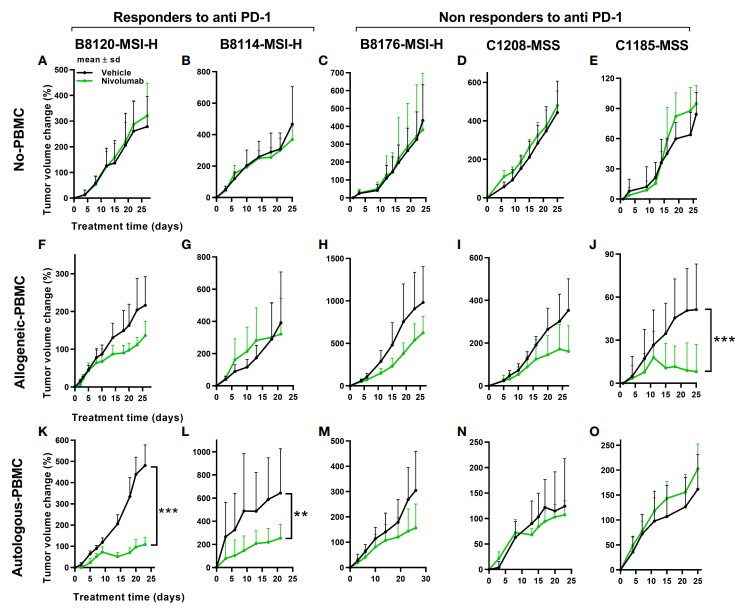 Fig.1 In vivo tumor responses to anti-PD-1 therapy under non-humanized, allogeneic, and autologous conditions.1
Fig.1 In vivo tumor responses to anti-PD-1 therapy under non-humanized, allogeneic, and autologous conditions.1
Q1: What makes PBMC humanized models superior to other humanized models?
A1: PBMC humanized models offer a rapid and cost-effective solution for reconstituting human immune cells in mice. Unlike CD34+ HSC models, they do not require months for immune system development, thus offering a quicker turnaround for preclinical studies. Furthermore, autologous PBMC models closely mimic individual patient responses, making them highly relevant for personalized medicine applications.
Q2: Can PBMC humanized models be used for long-term studies?
A2: While PBMC humanized models are primarily designed for short to medium-term studies, advancements in genetically modified mouse strains have extended their utility. For instance, the use of NSG-dKO mice minimizes GvHD, allowing for extended observational periods.
Q3: What types of cancer can be studied using Creative Biolabs' PBMC humanized models?
A3: Our PBMC humanized models are versatile and can be employed for a variety of cancers, including but not limited to breast, colon, esophageal, liver, lung, ovarian, prostate, and melanoma. The adaptability of our models allows for comprehensive cancer research across multiple tumor types.
The PBMC Immune System Humanized Model stands as a cornerstone in the preclinical investigation of cancer vaccines and immunotherapeutics. With its rapid engraftment, cost-effectiveness, and high relevance to human immune responses, this model offers unparalleled advantages for researchers. At Creative Biolabs, we are committed to advancing this technology, ensuring that our PBMC humanized models continue to provide reliable and insightful data to propel cancer research forward.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION