All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Creative Biolabs has accumulated rich experience through years of continuous exploration of tumor immunity and hereby launches the cytokine release syndrome (CRS) in vivo detection platform to help customers monitor CRS in CAR-T therapy to mitigate or prevent the formation of cytokine storms.
Although there is a high response rate after CAR-T cell infusion, safety concerns limit the use of CAR-T cell therapy. CRS is the most common immune-mediated toxicity in CAR-T cell therapy. Adverse responses of CRS are usually fever and constitutional symptoms, including stiffness, malaise, and anorexia. In addition, CRS can also cause systemic inflammation in some severe cases, including hypoxia, hypotension, organ dysfunction, etc. However, if the signs and symptoms of CRS are recognized and treated promptly, organ dysfunction can be prevented or reversed in most patients.
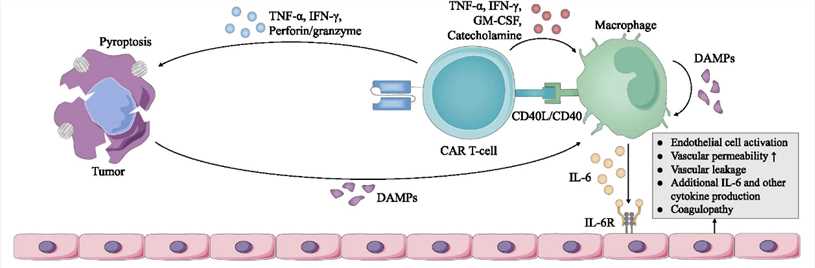 Fig.1 Cellular responses in CRS.¹
Fig.1 Cellular responses in CRS.¹
Creative Biolabs monitors the levels and changes of each cytokine associated with CRS through a combination of in vivo models and multiple in vitro cytokine analysis methods.
In Vivo Model
Creative Biolabs offers both syngeneic and xenograft models to help you achieve preclinical in vivo testing.
The syngeneic model is divided into (1) autologous transplantation, (2) allogeneic transplantation, and (3) allogeneic transplantation.
The xenograft model is divided into (1) cell line-derived xenograft (CDX) models, and (2) patients-derived xenograft (PDX) models.
All of the models we offered are performed strictly to the standard, using approved positive controls for dose-dependent testing. In addition, we can provide customized in vivo models for your project to meet your different needs in CAR-T cell therapy. You can click here to learn more about animal models.
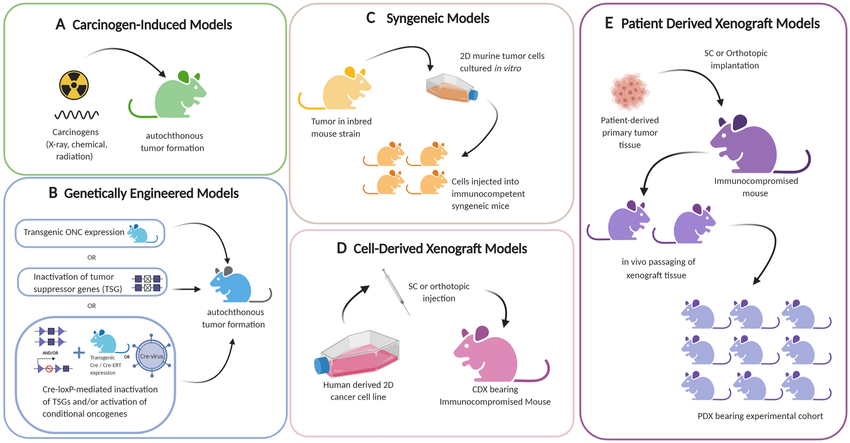 Fig.2 Preclinical in vivo mouse models of tumors.²
Fig.2 Preclinical in vivo mouse models of tumors.²
Mouse, rat, rabbit, non-human primate (NHP) models and more.
Cytokine Release Assay Service
In addition to in vivo models, Creative Biolabs offers a variety of cytokine analysis methods, including ELISA/CBA, intracellular cytokine staining (ICS), and ELIspot. By combining in vivo models with in vitro analysis methods, the level and changes of each cytokine can be monitored, which is conducive to early diagnosis, timely intervention and efficacy monitoring of CRS after CAR-T therapy.
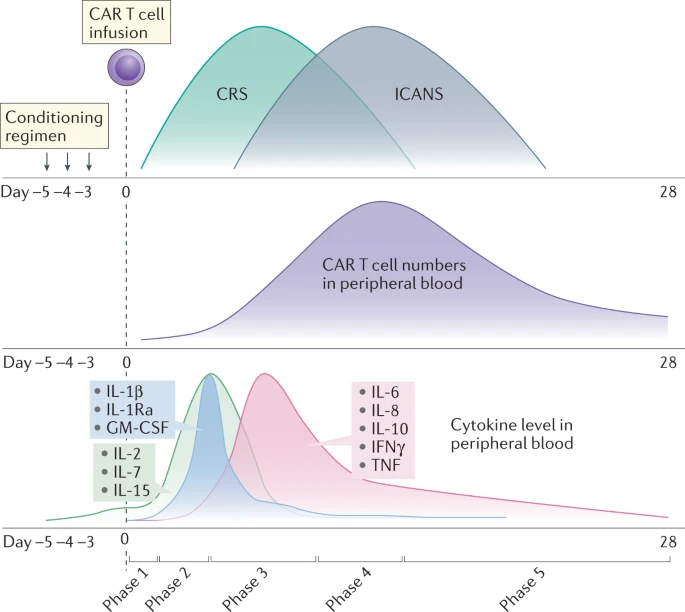 Fig.3 CAR-T cytokine levels in peripheral blood.³
Fig.3 CAR-T cytokine levels in peripheral blood.³
CRS can cause microvascular system damage, and a large number of cytokines such as IFN-γ and IL-6 are released, which can promote each other with the coagulation activation process, and finally form multi-organ failure. There are many cytokines associated with CRS, but the following are critical:
| IL-2 | Representing T cell activity, CAR-T releases IL-2 to maintain its own activity. High IL-2 levels indicate strong CAR-T activity. |
| IFN-γ | When CAR-T interacts with cancer cells, it releases IFN-γ to induce inflammation and activate monocytes and macrophages. The higher the IFN-γ level, the stronger the inflammatory response. |
| IL-6, IL-10 | When monocytes and macrophages are activated, they release large amounts of cytokines, resulting in CRS. Studies have shown that severe CRS after CAR-T therapy is associated with high levels of IL-6 and IL-10. |
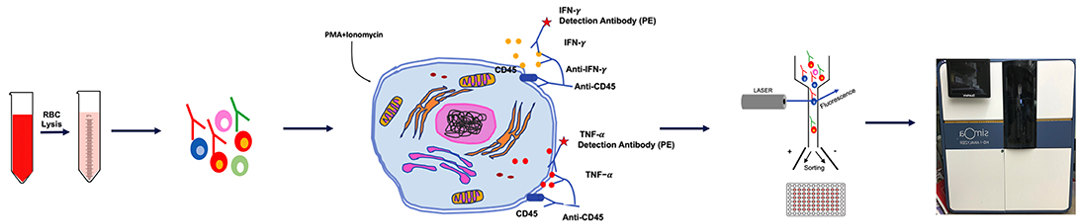 Fig.4 General flowchart for cytokine testing.⁴
Fig.4 General flowchart for cytokine testing.⁴
Creative Biolabs is able to design rigorous and detailed preclinical in vivo research experiments with appropriate in vivo animal models or customized models tailored to your needs. If you have project needs in this field, please contact us now. Creative Biolabs is committed to accelerating your research and providing comprehensive and reliable protocols.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION