This study introduces a novel method for predicting antigen-responsive single-domain antibodies (VHHs) by tracking the evolution of antibodies during immunization. The researchers utilized alpacas as a model, administering multiple injections of IgG fragments,
and employed high-throughput sequencing to monitor changes in the antibody repertoire. The details of the immunization procedure, including the immunization timeline and comprehensive preparation of immunogens for alpacas, are outlined in the experiment,
indicating the significance of properly designing immunization protocols and preparing immunogens for generating specific immune responses. The study revealed that antibody sequences exhibited substantial mutation accumulation and continuous turnover
throughout the immunization process. Based on these observations, researchers were able to predict VHH antibody clusters that reacted to the immunized antigen with a success rate exceeding 80%. This technique combines reasonable immunization strategies
with high-throughput sequencing to visualize antibody production and does not rely on in vitro screening, offering a new avenue for rapid antibody discovery.
1. How are VHHs generated through custom immunization services?
Custom immunization services involve immunizing camelids (such as llamas or alpacas) with a desired antigen to trigger an immunological response. Following immunization, blood samples are collected to extract RNA, convert into cDNA, and amplify the VHH genes. The VHH gene sequences are then cloned into appropriate vectors for library construction and screening.
2. How is the immune response monitored in camelids during the VHH generation process?
Camelids' immune responses are monitored by regularly collecting blood samples and evaluating the levels of specific antibodies against the target antigen. This is typically done using enzyme-linked immunosorbent assays (ELISAs) to measure antibody titers
in the serum. A strong and specific antibody response indicates effective immunization and helps determine the time of blood collection for future VHH library construction.
3. What criteria are used to select antigens for camelid immunization in VHH production?
Antigens chosen for camelid immunization must be highly immunogenic to generate a robust immune response. They can be proteins, peptides, whole cells, or other complex structures relevant to the research or treatment goal. The antigen should be well-characterized
and pure, although it can sometimes be presented as part of a complex to mimic its natural state. Factors such as antigen size, stability, and epitope accessibility are also important considerations.
4. What are the challenges associated with the custom immunization and VHH generation process?
Challenges in the custom immunization and VHH generation process include producing high-quality, immunogenic antigens, optimizing immunization techniques to elicit a strong immune response, and rapidly isolating and screening specific VHHs from large
libraries. Additionally, there may be technical issues in expressing and purifying VHHs in heterologous systems. Addressing the immunogenicity of camelid-derived VHHs in non-camelid species for therapeutic purposes is also critical.

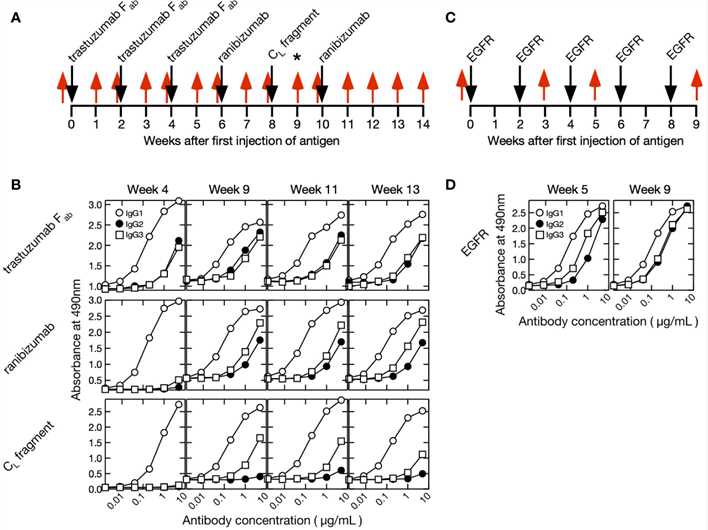 Fig. 1 Timeline and Antigens of the Alpaca Immunization Protocol.1
Fig. 1 Timeline and Antigens of the Alpaca Immunization Protocol.1