All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Chimeric antigen receptors (CARs), synthetic receptors designed to redirect immune cells towards specific tumor antigens, are a potent therapeutic tool with remarkable precision. CAR cell products, such as CAR T cells or CAR-engineered immune cells, involve the genetic alteration of T cells or natural killer (NK) cells to express these CARs. Incorporating CARs, immune cells can identify and engage with distinct antigens on tumor cells, thereby inducing targeted activation and destruction of cancerous cells. This utilization of CAR-modified immune cells signifies an innovative strategy within cancer immunotherapy, presenting tailored and precisely directed therapeutic avenues with the potential to achieve sustained treatment responses and improve patient outcomes. Ongoing research and clinical endeavors aim at refining CAR architecture, enhancing safety profiles, and extending the utility of CAR-based therapies across a diverse spectrum of cancer indications.
CAR design has undergone successive evolution phases, each introducing enhancements to augment efficacy, safety, and functionality in cancer immunotherapy.
The first-generation CARs comprise an antigen-binding domain, typically derived from a single-chain variable fragment (scFv) of an antibody and fused with a T cell activation domain like the CD3ζ chain. These constructs enabled T cells to recognize tumor antigens independently of major histocompatibility complex (MHC) presentation, eliciting T cell activation. However, the first-generation CARs exhibited limited persistence and efficacy, attributed to insufficient co-stimulation, resulting in inadequate T cell proliferation and persistence within the tumor microenvironment.
The second-generation CARs incorporated additional co-stimulatory domains such as CD28 or 4-1BB alongside the CD3ζ signaling domain. This dual signaling strategy significantly enhances CAR-T cell persistence, cytokine secretion, and anti-tumor cytotoxicity. Nonetheless, the second-generation CARs still face challenges like limited persistence, efficacy in solid tumors, and patient-specific variability.
The third-generation CARs further refine T cell activation by integrating two co-stimulatory domains, typically CD28 and 4-1BB, with the CD3ζ signaling domain. This multi-co-stimulatory approach synergistically enhances CAR-T cell activation, proliferation, and persistence within the hostile tumor microenvironment. However, the third-generation CARs are entangled by complexities in design and manufacturing, off-target toxicities, and limitations posed by the immunosuppressive tumor microenvironment.
The fourth-generation CARs, termed T cells redirected for universal cytokine-mediated killing (TRUCKs), are engineered to secrete immunomodulatory cytokines (e.g., IL-12) upon antigen recognition. TRUCKs serve to modulate the tumor microenvironment, improve T cell recruitment, and counteract immunosuppressive factors. Ongoing investigations into the fourth-generation CAR-T cell therapies aim to further optimize anti-tumor immune responses, particularly in solid tumors.
The evolution of CAR design from the basic first-generation constructs to sophisticated multi-co-stimulatory and cytokine-secreting CARs reflects a quest to refine T cell activation, persistence, and efficacy against cancer while mitigating off-target effects and adverse events. Future advances in CAR-T cell engineering will likely prioritize refining CAR architecture, improving safety profiles, and broadening the applicability of CAR-based therapies across diverse cancer types.
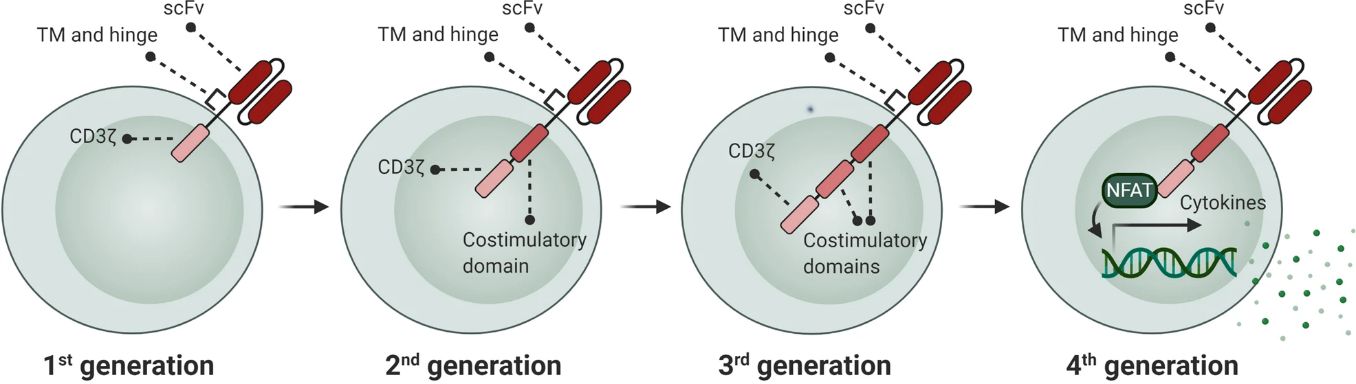 Fig. 1 Development of CAR generations. (Mansour, 2021)
Fig. 1 Development of CAR generations. (Mansour, 2021)
CAR technology enables the genetic modification of immune cells to express synthetic receptors tailored to recognize specific tumor-associated antigens (TAAs), triggering targeted immune responses against cancer cells. The engineered immune cell types utilized in CAR therapy include:
CAR-T cells
CAR-T cells are T lymphocytes that have been genetically engineered to express chimeric antigen receptors on their surface. These receptors are engineered to recognize TAAs expressed on tumor cells, leading to activation and targeted destruction of cancerous cells. CAR-T cell therapy is primarily employed in treating hematologic malignancies like B-cell acute lymphoblastic leukemia (B-ALL) and certain lymphomas.
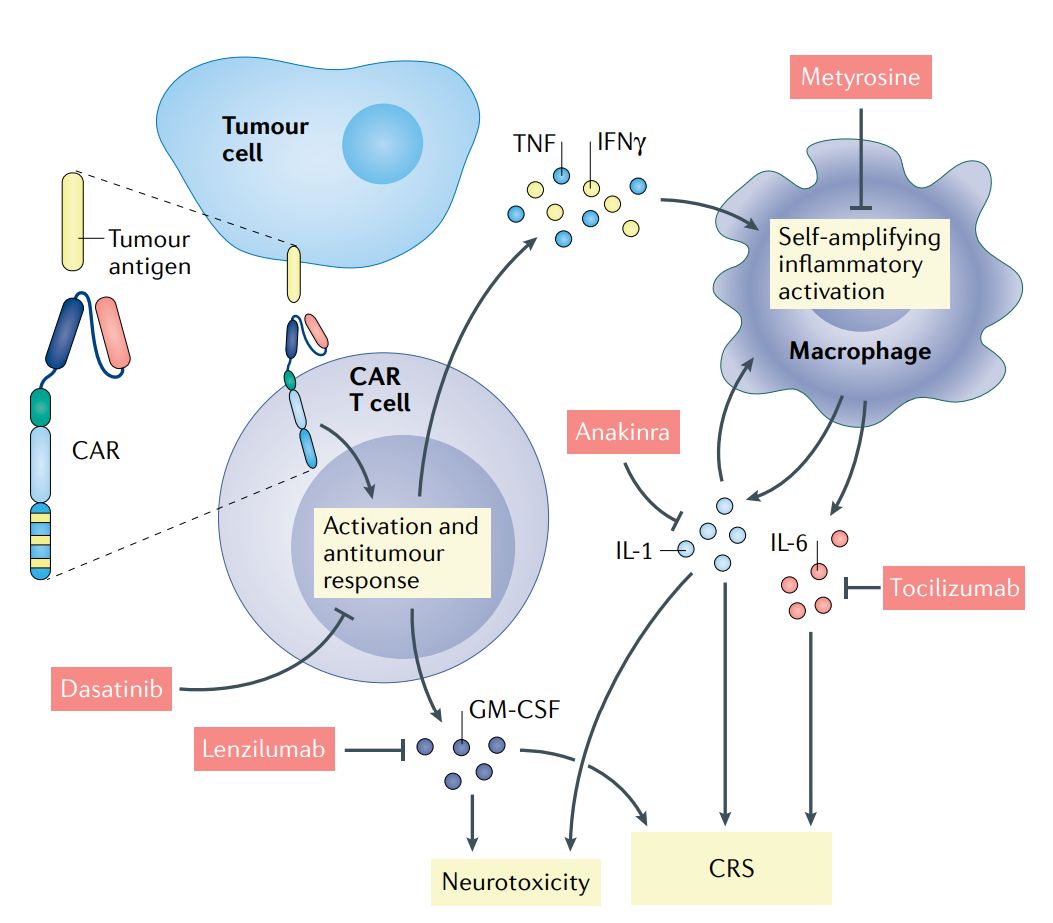 Fig. 2 Current molecular understanding and therapeutic intervention of CAR T cell-induced cytokine release syndrome and neurotoxicity (Rebecca, 2021)
Fig. 2 Current molecular understanding and therapeutic intervention of CAR T cell-induced cytokine release syndrome and neurotoxicity (Rebecca, 2021)
CAR-NK cells
CAR-NK cells are natural killer (NK) cells that have been modified to express chimeric antigen receptors. NK cells are innate immune effectors with cytotoxic activity against infected or malignant cells. By introducing CARs into NK cells, researchers aim to enhance their specificity and cytotoxic potency against tumor cells while preserving their inherent ability to recognize and eliminate abnormal cells.
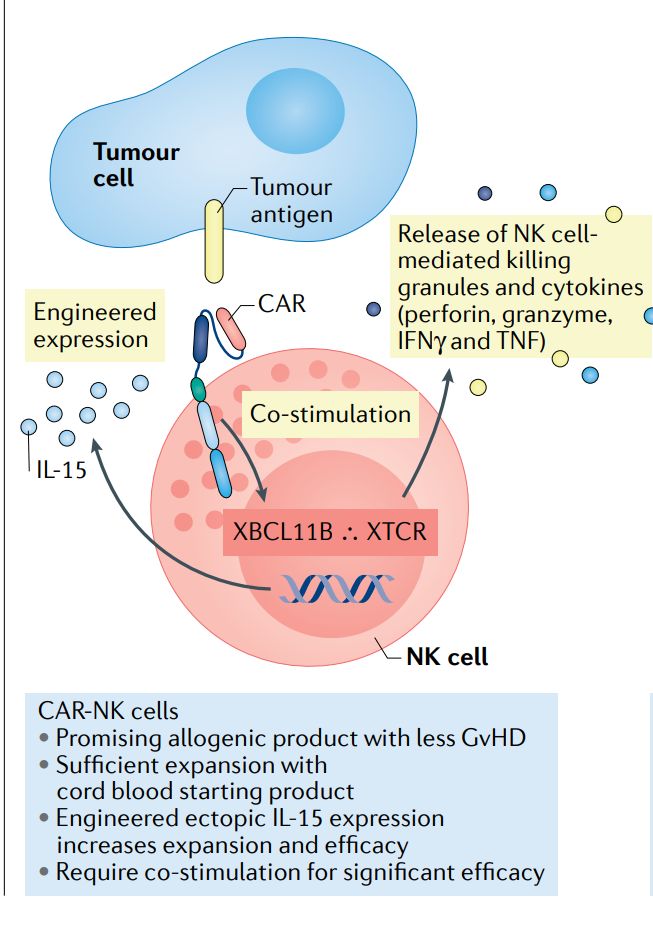 Fig. 3 Schematic of CAR-NK Cell (Rebecca, 2021)
Fig. 3 Schematic of CAR-NK Cell (Rebecca, 2021)
CAR-macrophages
Macrophages, as phagocytic immune cells, can also be engineered to express chimeric antigen receptors. CAR-modified macrophages are programmed to recognize and engulf cancer cells expressing specific TAAs, contributing to the anti-tumor immune response through antigen recognition and destruction.
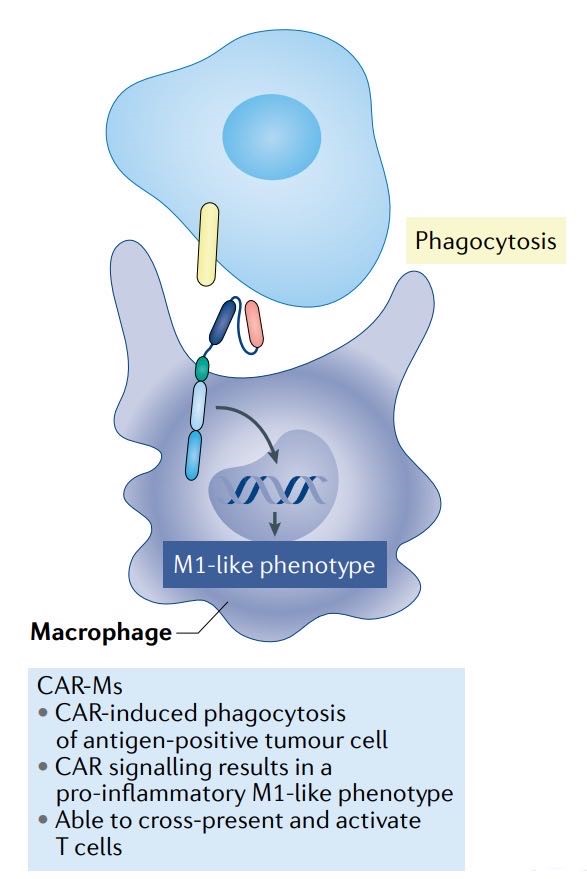 Fig. 4 Schematic of CAR-Macrophages (Rebecca, 2021)
Fig. 4 Schematic of CAR-Macrophages (Rebecca, 2021)
CAR-dendritic cells
Dendritic cells are specialized antigen-presenting cells crucial for initiating immune responses. CAR-modified dendritic cells can be engineered to express CARs targeting tumor-specific antigens, facilitating enhanced antigen presentation and activation of other immune cells against tumor cells.
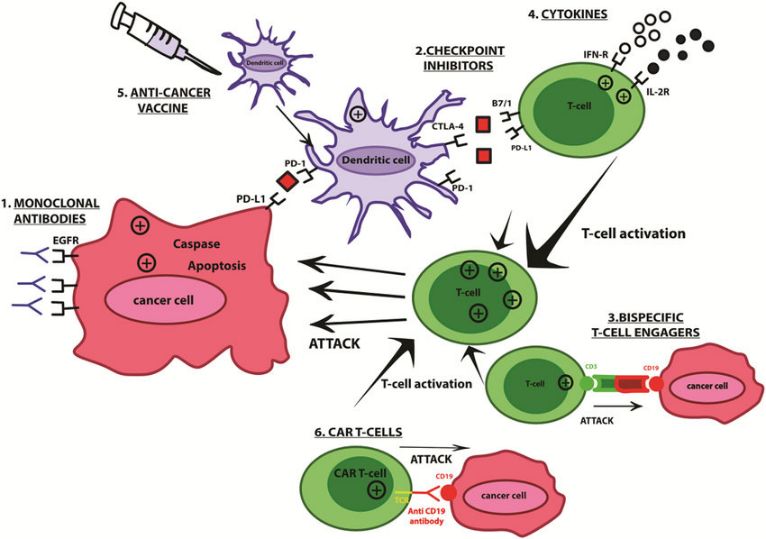 Fig. 5 Schematic of CAR-Dendritic Cells (Daniela, 2021)
Fig. 5 Schematic of CAR-Dendritic Cells (Daniela, 2021)
CAR-memory T cells
Memory T cells are a subset of T lymphocytes that persist long after initial immune responses, providing immunological memory against specific antigens. CAR-modified memory T cells are generated to enhance immune persistence and durability, potentially leading to sustained tumor control and the prevention of cancer relapse.
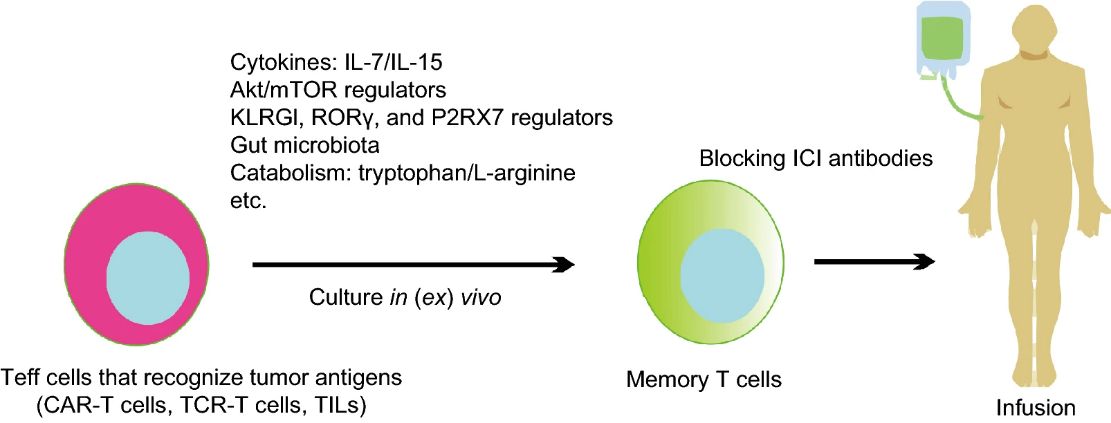 Fig. 6 Memory T cells: strategies for optimizing tumor immunotherapy (Qingjun, 2020)
Fig. 6 Memory T cells: strategies for optimizing tumor immunotherapy (Qingjun, 2020)
While CAR therapies have demonstrated remarkable success in treating certain cancers, they encounter significant challenges that warrant attention, like cytokine release syndrome (CRS), neurotoxicity, and antigen loss, which can compromise treatment efficacy and patient safety. To address these issues, various strategies have been employed, such as cytokine modulation to mitigate CRS and neurotoxicity and the incorporation of suicide genes into CAR constructs to enhance safety by allowing selective elimination of CAR-engineered cells if adverse events occur.
Moving forward, future directions in CAR therapy research involve refining CAR designs to optimize efficacy and safety profiles. Exploring allogeneic CAR therapies, which use donor-derived immune cells for broader patient applicability, is another promising attempt. Additionally, the development of off-the-shelf CAR products using universal donor cells that can be readily available for multiple patients aims to streamline CAR therapy accessibility and manufacturing.
Combination therapies represent another key focus for improving CAR therapy outcomes. Pairing CAR therapies with checkpoint inhibitors that release brakes on the immune system and adoptive cell therapies which trigger immune responses holds potential for enhancing anti-tumor efficacy and overcoming resistance mechanisms.
Creative Biolabs has been a reliable partner in providing CRO services for chimeric antigen receptor (CAR) therapy development for many academic research organizations and biotech companies. Some of the CAR therapies we are especially capable of developing include:
To make your work easier, all of our services are one-stop performed by our in-house scientists, such as Biomarker Identification & Selection, High Affinity ScFv Generation, Vector Design and Construction, Virus Packaging & CAR Gene Delivery, and various In Vitro & In Vivo Assays. We also provide CAR Vector Products, CAR Cell Products, and a proprietary online system for customizing CAR and CAR cell products, which offers full options to meet all unique needs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION