Cancer treatment has entered the era of immunotherapy from surgery, radiation and chemotherapy, which together constitute the four cornerstones of cancer treatment. The most successful applications of current immunotherapy are immune checkpoint inhibitors and adoptive cell therapy (ACT) of chimeric antigen receptor (CAR) T-cells. CAR cell therapy has made a major breakthrough in the treatment of hematological malignancies and is a hot spot in cancer treatment research. CAR is a recombinant receptor for antigen, which changes the specific characteristics and functions of immune cells in a single molecule, bypasses the barriers and incremental dynamics of active immunity, and rapidly generates tumor-targeting immune cells to kill tumor cells.
A chimeric antigen receptor (CAR) is a synthetic cell surface receptor that normally binds to a target cell surface antigen in an MHC-independent manner, thereby redirecting cytotoxic immune cells to target cells expressing the antigen for targeted killing of cancer cells. A CAR typically consists of four domains: the extracellular antigen-binding domain [single-chain variable fragment (scFv)], the spacer or hinge region, the transmembrane domain, and the intracellular signaling domain. The intracellular signaling domain has been extensively studied to generate CAR immune cells with the most active anti-tumor immunity. In principle, any cell surface molecule could be targeted by CAR, thus overcoming tolerance to self-antigens and antigen recognition gaps that limit the range of T-cell responses.
The structure of the three generations of CARs is mainly determined by the differences in the intracellular domains, especially the co-stimulatory domain (usually CD28, OX-40, or 4-1BB). First-generation CARs contain a single CD3ζ signaling domain and thus have limited activity in CAR T cells. Because no co-stimulatory signal is required, first-generation CARs have been used in CAR natural killer (NK) and CAR macrophages. The second-and third-generation CARs differ from the first-generation by the addition of one and two co-stimulatory signaling domains.
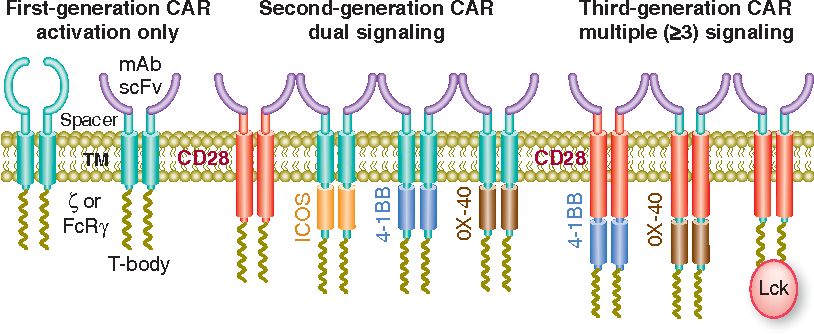 Fig. 1 Evolution of CAR design. (Sadelain, 2013)
Fig. 1 Evolution of CAR design. (Sadelain, 2013)
ACT refers to the isolation of active immune cells from patients, in vitro modification (or non-modification) and expansion, and reinfusion into patients to achieve the purpose of treating tumors. ACT therapy mainly includes NK, T-cell receptors, and CAR-T. ACT-based strategies to harness T cells for tumor destruction can be categorized into: (1) Tumor-infiltrating T cells were extracted from surgically resected tumor samples, expanded in vitro, and then reinfused into lymphodepleted patients. (2) T-cells are isolated from the peripheral blood of patients and expanded, and genetically modified to express T cell receptor (TCR) or CAR when reinfused into lymphodepleted patients. Through these two strategies, tumor cells can be specifically recognized and destroyed
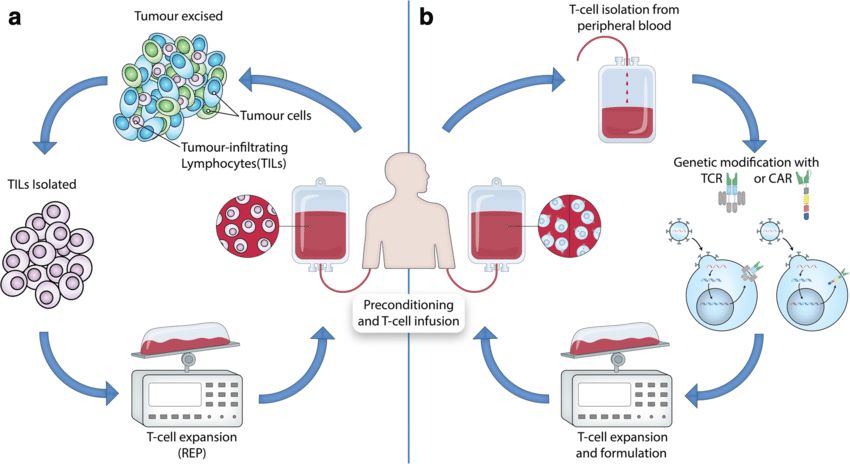 Fig. 2 Adoptive T-Cell therapy for cancer. (Met, 2019)
Fig. 2 Adoptive T-Cell therapy for cancer. (Met, 2019)
The use of T cell targeting vectors is expected to prevent the delivery of CAR to non-T cells, especially cancer cells, avoiding fatal consequences for patients. The CAR vector system includes lentiviral vector (LV), adenovirus-associated vector (AAV), synthetic polymer nanocarrier (NC), and lipid nanoparticle (LNP). The rapid development of the vector system allows CAR-T cells to generate and eliminate tumor cells in vivo, which is a milestone for CAR therapy and gene therapy. However, unresolved questions include: short-, medium-, and long-term safety and efficacy of permanent and transient transfer methods. Optimizing and adjusting protocols to minimize inflammation and immune-mediated particle dose loss associated with vector administration while maintaining lymphocyte viability and activity are urgent issues to be addressed.
3.1 LV
LVs are usually pseudo-typed with glycoprotein G of vesicular stomatitis virus (VSV-G), forming particles with a diameter of 120-150 nm, and VSV-G mediates cell entry through the low-density lipoprotein receptor and its family members to achieve high transduction efficiency. CAR therapy mainly relies on lentiviral or γ-retroviral vectors for in vitro transduction. In 2018, 54% of US clinical studies used LVs for CAR-T cell generation. Previous studies have shown that LVs targeting receptors not only mediate high target cell selectivity but also transfer activating stimuli to target cells. The advantage is the stable integration of the CAR expression cassette, high transduction efficiency, and relatively uniform gene copy number, but there are also defects such as limited vector capacity and risk of insertion mutation.
3.2 AAV
AAVs consist of a non-enveloped protein capsid containing a single-stranded DNA genome, and its genetic modification is usually transient. After interacting with their target receptors, clathrin-mediated endocytosis, and intracellular trafficking, AAVs enter the nucleus and release single-stranded transgenes that, following second-strand synthesis, are available for transcription. Targeting at the N-terminus greatly reduces the hepatic accumulation of intravenously administered vectors.
3.3 LNP and NC
Currently, non-viral vectors include LNPs and NCs. Unlike complex viral mechanisms, processes such as cell entry, trafficking, and transgene delivery of nonviral vectors are all mediated by the physicochemical properties of the particle and its payload. NCs are complexes of positively charged poly (β-amino ester) polymers and negatively charged nucleic acids. After being absorbed by the cell, the nucleic acid of NC will escape from the inclusion body and enter the cell. In LNP, the mRNA is encapsulated in the core part of the lipid particle through electrostatic interaction, which is taken up by the host cell and escapes from the endosome, and the mRNA can be used for translation before it is degraded. Appropriate biological modifications can address RNA biostability and immunogenicity issues, including adding 5' cap analogs and 3' poly-A tails, introducing stabilizing sequences in the 5' and 3' untranslated regions, Codon optimization of transcription, and use of less immunogenic modified nucleotides.
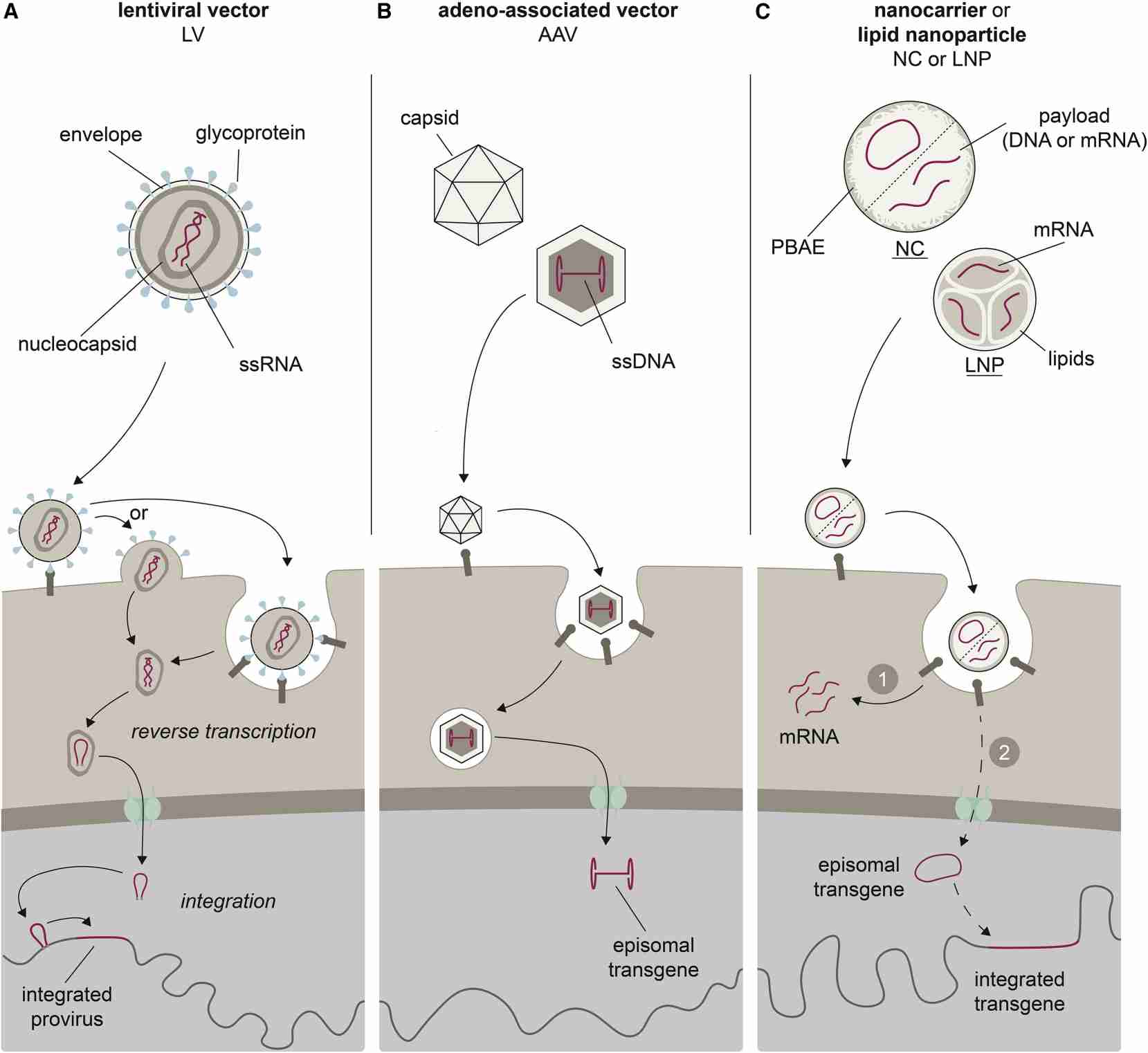 Fig. 3 Vector system for construction of CARs. (Michels, 2022)
Fig. 3 Vector system for construction of CARs. (Michels, 2022)
Common immune cell types for CAR-modified immune cell therapy include T cells, NK cells, and macrophages.
Till now, five CAR-T cells have been approved by the US Food and Drug Administration for the treatment of hematological malignancies, and they all target B cell surface markers, four of which target CD19, while one targets B-cell maturation antigen (BCMA). B cell maturation antigen (BCMA). However, CAR T-cell therapy is still challenged by many factors, such as patient disease progression, insufficient T cell harvest, delayed CAR cell manufacturing, low CAR cell yield, inherent T cell defects, etc.
(1) Tisagenlecleucel (Kymriah™) is the FDA-approved CAR-T therapy for the treatment of patients ≤25 years with refractory or second or later relapsed precursor B-cell acute lymphoblastic leukemia on August 30, 2017. And on May 1, 2018, it was approved for relapsed or refractory large B-cell lymphoma, including diffuse large B-cell lymphoma (DLBCL), high-grade B-cell lymphoma, and follicular lymphoma Caused DLBCL;
(2) Axicabtagene ciloleucel (Yescarta™) is the second CAR-T therapy approved by the FDA on October 18, 2017, for large B-cell lymphoma after at least second-line systemic therapy, including DLBCL, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma;
(3) Brexucabtagene autoleucel (Tecartus™), the third CAR-T therapy targeting CD-19, was approved by the FDA on July 24, 2020 for relapsed or refractory mantle cell lymphoma;
(4) Lisocabtagene maraleucel (Breyanzi™) is also a CD19-targeted CAR T therapy approved for relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy, including DLBCL (including DLBCL caused by indolent lymphoma), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B;
(5) Idecabtagene vicleucel (Abecma®) is the only CAR-T therapy that does not target CD19, but instead targets BCMA. Approved by the FDA on March 26, 2021 for relapsed or refractory multiple myeloma after four or more lines of systemic therapy, including a proteasome inhibitor, an immunomodulatory agent, and an anti-CD38 monoclonal antibody.
NK cells are functionally similar to CD8+ cytotoxic T cells and kill target cells through similar cytotoxic mechanisms, but lack somatic rearrangements and antigen-specific TCRs. The killing of target cells by NK cells depends on a balance between inhibitory and activating signals. CAR-modified NK cells could effectively eradicate some heterogeneous tumors in which tumor cells do not express CAR-targeted antigens through CAR-dependent and NK cell receptor-dependent mechanisms (CD16-mediated antibody-dependent cell-mediated cytotoxicity). The lack of an effective gene transfer method in primary NK cells is one of the major obstacles in NK cell immunotherapy. NK cells make up only a small fraction of peripheral blood leukocytes, so generating sufficient numbers of them is another major challenge. Compared with CAR-T cells, CAR-NK cells have some significant advantages, including (1) Better safety and lower toxicity: Due to the limited lifespan of CAR-NK cells in circulation, the risk of on-target/off-target tumor toxicity to normal tissues is relatively low. Little to no CRS and neurotoxicity, also reduces the risk of graft-versus-host disease (GVHD); (2) Multiple mechanisms to activate cytotoxic activity; (3) CAR - NK cells can be engineered to target multiple antigens, enhance proliferation and persistence in vivo, increase infiltration into solid tumors, overcome resistant tumor microenvironment, and ultimately achieve effective antitumor responses. Although CAR-NK therapy has the above-mentioned benefits over CAR-T therapy, almost all the limitations associated with CAR-T therapy also apply to CAR-NK cells.
Results from the first CAR-NK cell trial (NCT03056339) showed safety and favorable clinical activity in patients with CD19+ non-Hodgkin's lymphoma or chronic lymphocytic leukemia. However, so far only limited progress has been made in the field of CAR-NK cells, and some clinical trials are still in progress. With the increase of safety and good activity in preclinical research and clinical trials, it is expected that there will be breakthroughs in the development of CAR-NK cell therapy for cancer.
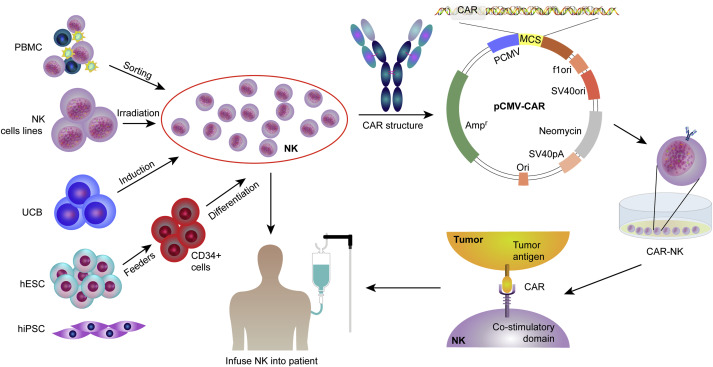 Fig. 4 Procedures for clinical application of CAR-NK therapy. (Wang, 2020)
Fig. 4 Procedures for clinical application of CAR-NK therapy. (Wang, 2020)
CAR-Macrophage Therapy
CAR-macrophages as an alternative therapy for CAR-T and CAR-NK cell therapy-related disorders. CAR-macrophages share many features and obstacles with CAR-T cells, such as the need for specific antigens, antigen escape and downregulation, and systemic cytokine toxicity. Compared with CAR-T cells in solid tumors, CAR-macrophages have unique advantages in immune cell suppression and expansion into the TME, as well as immunosuppressive TME. A major advantage of using macrophages for ACT is their propensity to migrate and expand into tumors. The focus of CAR- macrophage research is to activate and enhance phagocytosis through different intracellular domains. CD19 and HER2 are common tumor targets of CAR-macrophage. Researches on CAR macrophages are mainly in the preclinical stage. As of November 2020, two clinical trials based on the CAR-macrophages strategy have been approved by the FDA.CARISMA's CT-0508 drug uses anti-HER2 CAR macrophages to treat patients with relapsed/refractory HER2-overexpressing tumors (Phase I clinical trial). The other is MaxCyte's MCY-M11, which uses mRNA-targeted PBMCs (including CAR-macrophages) to express mesothelin-CAR for the treatment of patients with relapsed/refractory ovarian cancer and peritoneal mesothelioma.
Creative Biolabs has been a reliable partner in providing CRO services for chimeric antigen receptor (CAR) therapy development for many academic research organizations and biotech companies. Some of the CAR therapies we are especially capable of developing include:
To make your work easier, all of our services are one-stop performed by our in-house scientists, such as Biomarker Identification & Selection, High Affinity ScFv Generation, Vector Design and Construction, Virus Packaging & CAR Gene Delivery, and various In Vitro & In Vivo Assays. We also provide CAR Vector Products, CAR Cell Products, and a proprietary online system for customizing CAR and CAR cell products, which offers full options to meet all unique needs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION