All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
In the advancing field of cancer immunotherapy, the development of checkpoint-modulated synthetic Tumor-Infiltrating Lymphocytes (TILs) has emerged as a groundbreaking innovation. Leveraging over two decades of experience, Creative Biolabs is at the forefront, providing cutting-edge services that integrate the intricacies of synthetic biology with immunotherapy to enhance therapeutic efficacy against various tumors, particularly metastatic melanoma.
Cancer immunotherapy has revolutionized treatment paradigms by harnessing the body's immune system to target and destroy cancer cells. The failure of T-cells in the immunosuppressive tumor microenvironment has been a significant barrier; however, the modulation of immune checkpoints has provided a means to rejuvenate these fatigued immune cells. Checkpoints such as PD-1 and CTLA-4 act as immune brakes. Through checkpoint inhibitors, these brakes are released, allowing T-cells to regain functionality and target cancer cells effectively. Clinical trials have demonstrated that targeting PD-1 alone can result in tumor regression in approximately 30-40% of patients, highlighting the therapeutic potential of this approach but emphasizing the need for enhanced strategies to increase response durability and rates.
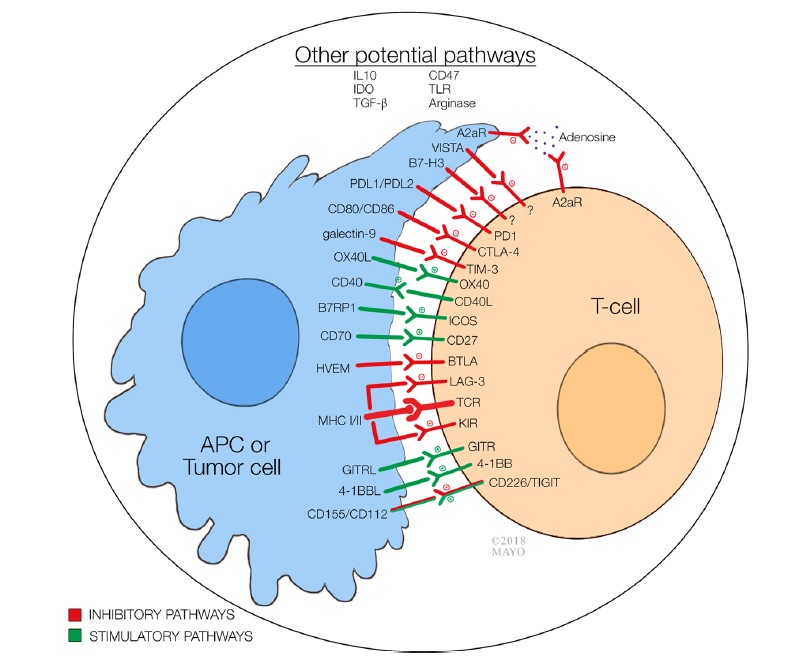 Fig.1 Immune checkpoints involved in tumor microenvironment.1,2
Fig.1 Immune checkpoints involved in tumor microenvironment.1,2
Creative Biolabs offers a bespoke Checkpoint Modulated Synthetic TIL Development Service that combines state-of-the-art gene editing techniques with the latest advancements in TIL therapy. Our service is designed to engineer TILs that are not only tumor-reactive but also resistant to immunosuppressive signals within the tumor microenvironment. By incorporating advanced gene editing technologies, we conduct precise modifications at checkpoints locus to create Checkpoints knockout TILs, enhancing their antitumor efficacy and persistence.
Creative Biolabs provides a comprehensive suite for the development of synthetic TILs tailored to exploit checkpoint modulation. Our services include:
We begin by isolating TILs from fresh tumor samples and implementing a rapid expansion protocol (REP) to boost cell numbers while maintaining viability.
Utilizing advanced gene editing technologies, we precisely edit genes coding for inhibitory checkpoint receptors, such as PD-1, to engineer TILs with enhanced antitumor activities.
Post-editing, we conduct in-depth functional assays including cytokine profiling and cytotoxic assays against antigen-pulsed target cells to evaluate TIL efficacy.
We employ off-target analysis using the latest sequencing technologies to ensure the genetic modifications are precise and safe for clinical applications.
At Creative Biolabs, our service stands out due to its precision and innovative approach:
Q1: How does checkpoint modulation improve TIL efficacy?
A1: Checkpoint modulation, particularly PD-1 knockout, reverses TIL exhaustion by blocking inhibitory signals within the tumor microenvironment. This genetic intervention enhances TIL cytotoxicity and cytokine secretion, essential for effective tumor clearance.
Q2: What types of tumors can benefit from this service?
A2: Our Checkpoint Modulated Synthetic TIL Development Service is adaptable to a broad range of tumors, expanding the therapeutic applicability of this technology.
Creative Biolabs' Checkpoint Modulated Synthetic TIL Development Service offers a powerful platform for enhancing cancer immunotherapy. With a blend of precision gene-editing and deep scientific expertise, we are committed to advancing the therapeutic potential of TILs, delivering optimized solutions for clients worldwide.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION