All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
The advancement of cancer immunotherapy represents a paradigm shift in oncology, offering new hope for the treatment of various malignancies. Among these cutting-edge therapies, synthetic tumor-infiltrating lymphocytes (TILs) have emerged as potent agents in combating cancer. These engineered immune cells provide targeted, robust responses against tumors, overcoming some traditional treatment challenges. At Creative Biolabs, we are committed to advancing synthetic TIL technologies, representing a breakthrough in personalized cancer therapy.
Creative Biolabs offers comprehensive synthetic TIL development services designed to enhance the therapeutic potential of TILs in oncology. Synthetic TILs, distinguished by their genetic modifications, are engineered to target tumors with high specificity and reduced off-target effects. These tailored TILs exhibit enhanced functionality compared to their natural counterparts, offering greater efficacy in eliminating cancer cells, particularly in solid tumors where traditional immunotherapies face limitations.
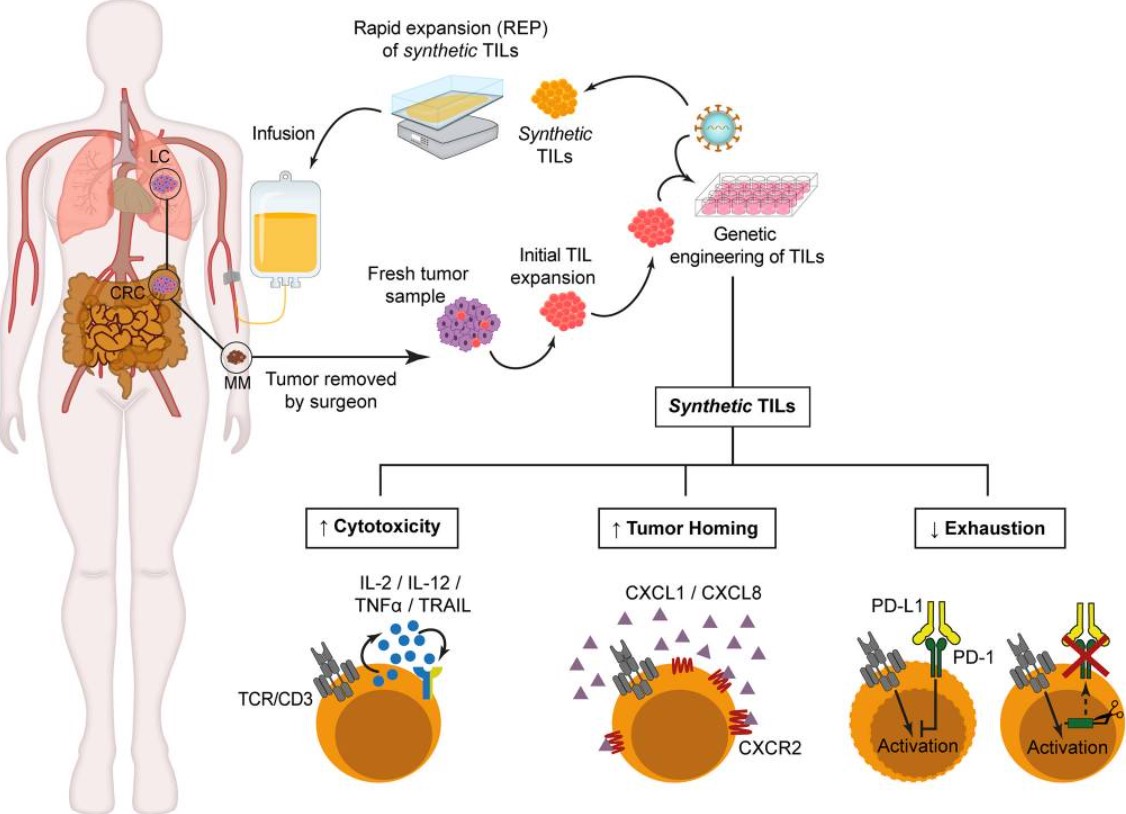 Fig.1 Synthetic TILs manufacture.1,2
Fig.1 Synthetic TILs manufacture.1,2
The tumor microenvironment often employs immune checkpoints to evade immune destruction. Our Checkpoint Modulated Synthetic TIL Development Service addresses this by integrating checkpoint inhibition mechanisms within TILs. Through genetic engineering, TILs are modified to resist immunosuppressive signals, such as PD-1 and CTLA-4, thereby maintaining their cytotoxic activity in the tumor milieu. This service enhances the persistence and functionality of TILs, which is crucial for sustained therapeutic efficacy.
TILs exert potent cytotoxic effects to effectively eliminate tumor cells. At Creative Biolabs, we enhance TIL cytotoxicity by introducing modifications such as TRAIL and modified TNF-alpha, which improve the tumor cell-killing capabilities of these lymphocytes. Enhanced cytotoxic TILs are especially effective against tumors with high antigenic expression, providing a powerful tool for cancer eradication.
The efficacy of TIL therapies hinges significantly on the ability of the lymphocytes to home to tumor sites. Our Tumor Homing Ability Enhanced Synthetic TILs are genetically modified to express chemokine receptors like CXCR2, which are pivotal in guiding these cells to tumor sites. This strategic enhancement increases cell infiltration into the tumor stroma, facilitating a localized, potent immune response.
T cell exhaustion serves as a substantial barrier to effective TIL therapy. By employing gene-editing technologies, we can reduce the expression of exhaustion markers like PD-1 in synthetic TILs. This service ensures prolonged T cell activity and enhanced tumor clearance, making this approach highly suitable for patients with chronic infections or high tumor burdens.
Our development service workflow is meticulously designed to ensure optimal results:
Tumor-specific antigens are identified for precise targeting, followed by isolation of TILs from patient-derived samples.
TILs undergo genetic modifications to integrate specific functional enhancements, depending on the desired therapy aspect.
Modified TILs are expanded under controlled conditions to achieve the required therapeutic dose.
Rigorous testing ensures the purity, potency, and safety of the synthetic TILs before clinical application.
Synthetic TILs are applicable in diverse oncology contexts:
Q1: Are there specific tumor types that benefit most from Synthetic TILs?
A1: While effective across a range of malignancies, Synthetic TILs show particularly promising results in aggressive and treatment-resistant cancers such as metastatic melanoma.
Q2: Can we integrate multiple enhancements in a single TIL product?
A2: Yes, synthetic TILs can be engineered to incorporate various functional enhancements, allowing for a synergistic approach to cancer therapy.
By leveraging our scientific expertise and cutting-edge technology, Creative Biolabs is poised to propel synthetic TIL therapies to the forefront of cancer treatment strategies, providing hope and enhanced outcomes for patients worldwide.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION