All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
In recent years, immunotherapy has revolutionized the treatment of numerous cancers, introducing unprecedented strategies aimed at harnessing the body's immune system to fight malignant cells. T cell exhaustion has emerged as a significant barrier to the efficacy of these therapies, presenting unique challenges that stifle immune response, particularly within the tumor microenvironment (TME). At Creative Biolabs, we are committed to advancing the frontier of adaptive immunotherapy through innovative approaches designed to mitigate T cell exhaustion, thus enhancing the development of synthetic tumor-infiltrating lymphocytes (TILs).
T cell exhaustion is characterized by the progressive loss of T cell effector functions, marked by poor cytotoxic capability and increased expression of inhibitory receptors like PD-1, CTLA-4, and TIM-3. These inhibitory signals, amplified in the TME, lead to the functional impairment of T cells, thwarting durable anti-tumor responses. At Creative Biolabs, we leverage advanced gene-editing technologies and checkpoint blockade strategies to reverse T-cell exhaustion.
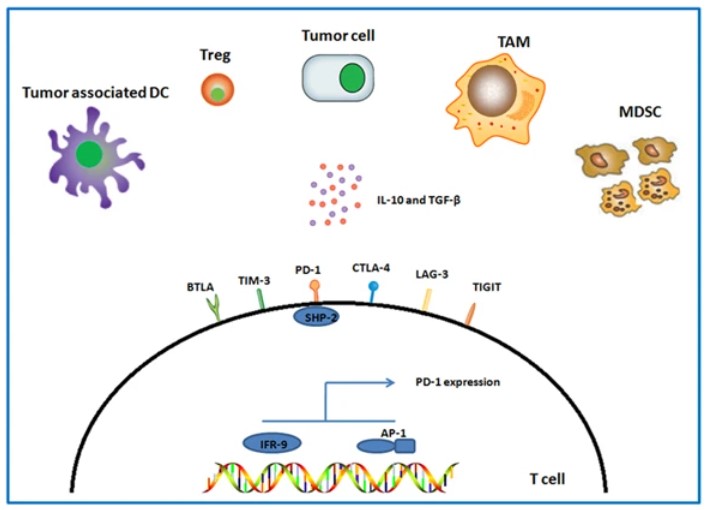 Fig.1 Potential inhibitory receptors involved in T-cell exhaustion.1,2
Fig.1 Potential inhibitory receptors involved in T-cell exhaustion.1,2
Creative Biolabs focuses on the generation of synthetic TILs that have reduced susceptibility to exhaustion. We employ cutting-edge genetic engineering to modify the TILs at a molecular level, enhancing their persistence and functionality within the harsh milieu of the TME. By curbing the expression of exhaustion markers, our synthetic TIL development service aims to prolong the efficacy of TIL therapies across a diverse range of solid tumors. This approach enhances TIL resilience and empowers them to mount robust anti-cancer attacks over a sustained period.
Central to our approach is targeting the molecular pathways responsible for T-cell exhaustion. We employ strategies that include:
Focusing on PD-1 and CTLA-4 pathways to sustain T cell activation.
Enhancing the metabolic pathways to bolster T cell energy and persistence.
Modulating gene expression patterns linked to exhaustion, thereby supporting T cell vigor and longevity.
Q1: What specific targets are modified in synthetic TILs to reduce exhaustion?
A1: Synthetic TILs are engineered to modulate key inhibitory checkpoints such as PD-1, CTLA-4, TIM-3, and LAG-3. Advanced gene-editing technologies are employed to achieve precise alterations that reinstate TIL functional capabilities.
Q2: How do synthetic TILs differ from conventional TIL therapies?
A2: Conventional TIL therapies often struggle with sustaining T cell activity within the tumor's immunosuppressive microenvironment. In contrast, synthetic TILs are crafted to maintain high proliferative potential and effector function through strategic gene modifications that pre-empt and alleviate exhaustion pathways.
Creative Biolabs remains at the forefront of T-cell therapy innovation, providing solutions that empower the next generation of cancer treatments. Our commitment to overcoming the challenges posed by T-cell exhaustion is unwavering, made possible through our sophisticated development services and thorough scientific grounding.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION