All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
CAR (Chimeric Antigen Receptor) constructs are synthetic receptors utilized in immunotherapy, specifically within CAR T-cell therapy. These synthetic receptors comprise distinct structural components with specialized functions. The Extracellular Domain incorporates an antigen-binding site derived from a single-chain variable fragment (scFv), sourced from an antibody, enabling precise targeting of tumor antigens on cancer cells. The Spacer/Hinge region connects the antigen-binding domain to the Transmembrane Domain, facilitating flexible for effective binding interactions. Anchoring the CAR within the T cell membrane, the Transmembrane Domain ensures its stability and functionality. Intracellular Signaling Domains play a crucial role in initiating T cell activation upon antigen recognition. Typically, CARs contain one or more signaling domains such as CD3ζ (from the T cell receptor complex) and costimulatory domains like CD28 or 4-1BB (CD137). Upon binding to the target antigen, the CAR initiates T cell activation, resulting in the secretion of cytokines (e.g., interferon-gamma, interleukins) that recruit other immune cells and bolster the anti-tumor response. Additionally, activated CAR T cells directly eliminate cancer cells, and their persistence post-treatment offers prolonged surveillance against cancer recurrence.
Retroviral vectors are employed as gene delivery systems in CAR (Chimeric Antigen Receptor) T-cell therapy, ensuring the integration of CAR genes into the host cell genome for sustained expression of the Chimeric Antigen Receptor. Retroviruses, belonging to the RNA virus family, possess the capability to integrate their genetic material into the DNA of host cells. Retroviral vectors, which are engineered derivatives of these viruses, have been adapted to deliver specific genes into target cells.
In CAR T-cell therapy, retroviral vectors are utilized to transfer the gene encoding the Chimeric Antigen Receptor into T cells. Typically, this CAR gene comprises sequences encoding the extracellular antigen-binding domain (often a single-chain variable fragment, scFv), a transmembrane domain, and intracellular signaling domains. T cells are isolated from the patient's blood and exposed to the retroviral vector containing the CAR gene. Upon infection, the retroviral vector delivers of the CAR gene into T cells' genome.
Within the T cell, the retroviral vector's genetic material, including the CAR gene, undergoes transcription into DNA by the enzyme reverse transcriptase. The resulting DNA is then integrated into the host cell's genome by the viral integrase enzyme, establishing the CAR gene as a permanent component of the T cell's genetic material.Through cell division and proliferation, the CAR gene is transmitted to daughter cells, ensuring sustained expression of the Chimeric Antigen Receptor.
The integration of the CAR gene empowers the T cells to produce the CAR protein on their surface. As a result, these engineered CAR T cells gain the ability to identify and bind to specific antigens on cancer cells. Upon encountering cancer cells expressing the target antigen, the CAR is activated, triggering T cell activation and subsequent elimination of the cancer cells through cytokine release and direct cytotoxic effects.
Retroviral vectors are favored in CAR T-cell therapy owing to their ability to achieve stable integration of the CAR gene into T cells, thereby ensuring enduring expression of the Chimeric Antigen Receptor and sustained anti-tumor activity. Nonetheless, the use of retroviral vectors necessitates careful consideration of safety concerns, including the potential risk of insertional mutagenesis and potential off-target effects, which continue to be areas of active research and development in the domains of gene therapy and immunotherapy.
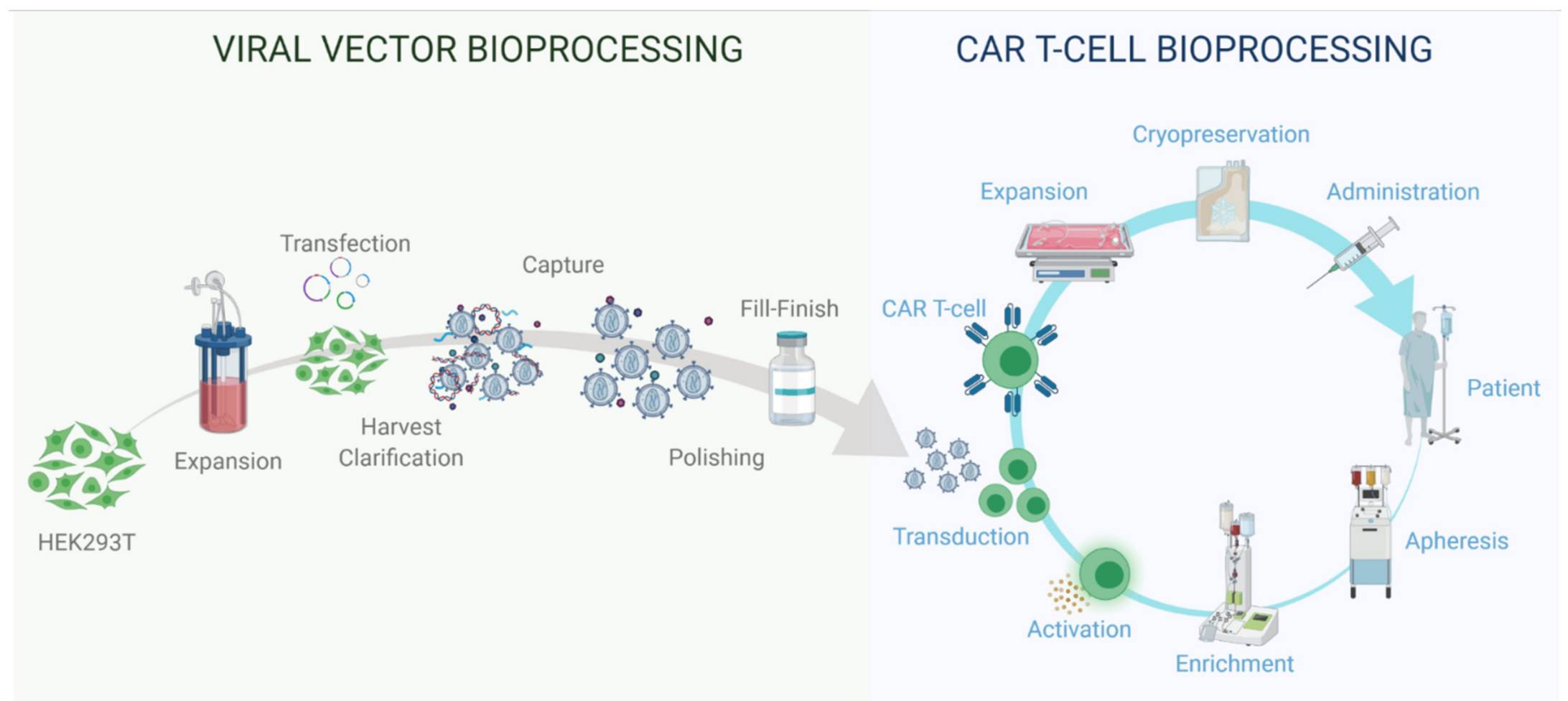 Fig 1. Viral Vector and Autologous CAR T Cell Therapy Bioprocessing (Roman, 2021)
Fig 1. Viral Vector and Autologous CAR T Cell Therapy Bioprocessing (Roman, 2021)
Non-viral vectors represent a varied assortment of gene delivery systems employed for introducing genetic material into cells without viral elements. Unlike viral vectors, which encompass retroviruses and adenoviruses, non-viral vectors are typically synthetic or natural formulations. They are favored for their enhanced safety profile, simple design, and reduced risk of eliciting immune responses or causing insertional mutagenesis.
Among non-viral vectors, plasmid DNA vectors stand out as circular DNA molecules engineered to harbor specific genes, including therapeutic genes such as those encoding Chimeric Antigen Receptors (CARs). Plasmid DNA can be introduced into cells using diverse methods, including electroporation, microinjection, or lipid-based transfection reagents. These techniques enable efficient delivery of genetic material into target cells, facilitating the expression of desired genes for therapeutic applications. The use of non-viral vectors represents a promising avenue in gene therapy, offering safety and flexibility advantages over viral-based approaches.
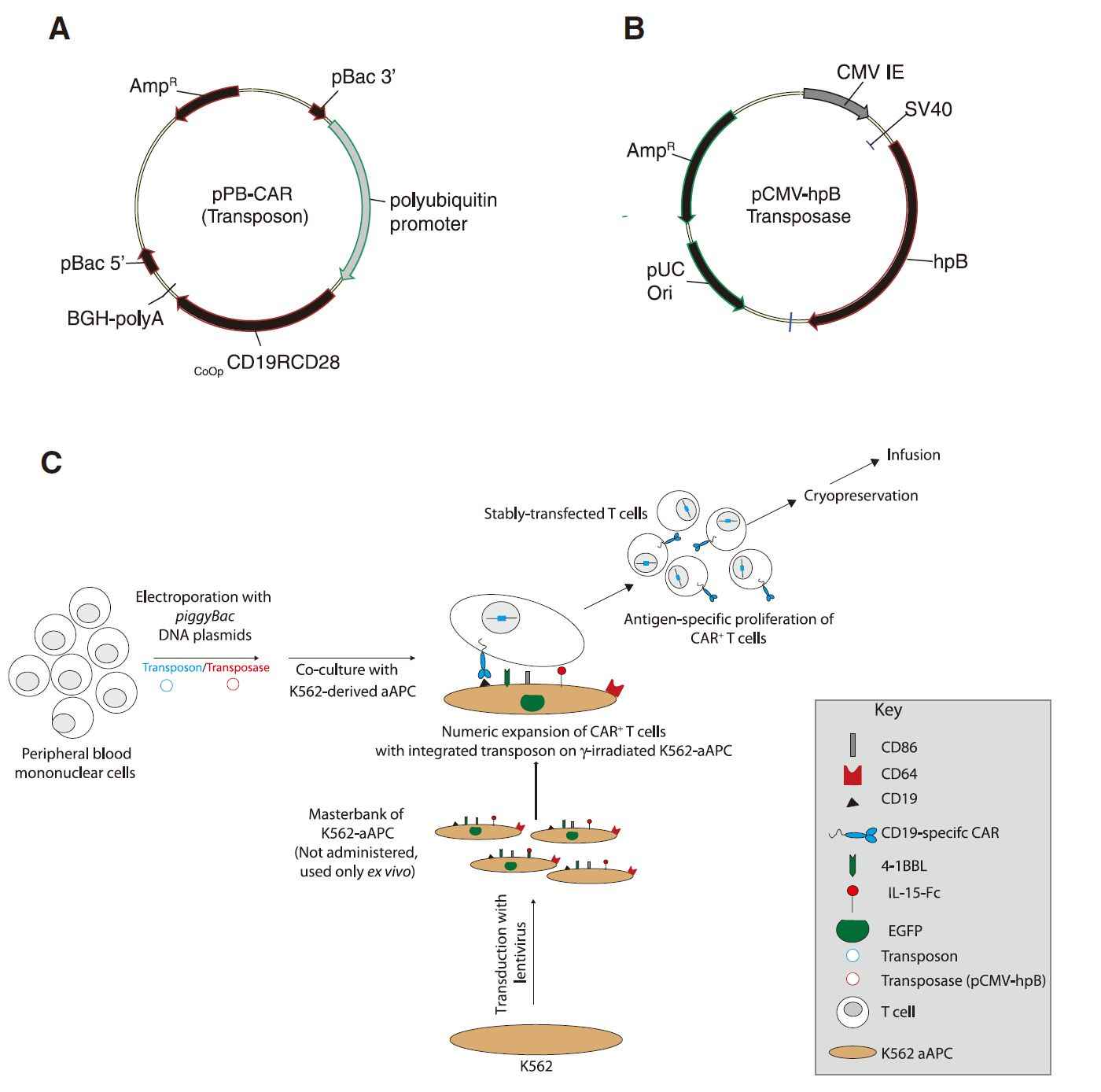 Fig 2. Schematic of the Two PB DNA Plasmids Electrotransferred (Pallavi, 2010)
Fig 2. Schematic of the Two PB DNA Plasmids Electrotransferred (Pallavi, 2010)
CAR T-cell therapy has demonstrated notable efficacy in treating specific hematologic malignancies, such as B-cell leukemia and lymphoma. Regulatory agencies have approved several CAR T-cell therapies for clinical application, underscoring its promise in cancer treatment. Vector systems play a crucial role in generating clinical-grade CAR-T cell products for patient therapy, facilitating scalable manufacturing processes and ensuring consistent CAR expression and potency. These vector technologies are essential for producing standardized and effective CAR-T cell therapies, supporting their translation from research settings to clinical practice. Ongoing advancements in vector systems contribute to optimizing CAR T-cell therapy and expanding its therapeutic impact in oncology.
Empowered by our state-of-the-art CellRapeutics™ Chimeric Antigen Receptor (CAR) Technology, Creative Biolabs offers world-leading CAR-T therapy development services, aiming to improve the ability of CAR cells to attack solid tumors. Our one-stop services covering Biomarker Identification & Selection, ScFv Generation, CAR Design & Construction, Virus Packaging & CAR Gene Delivery, CAR-T In Vitro Assay, CAR-T Preclinical In Vivo Assay, and CAR-T Clinical Trial. We also provide CAR Vector Products, CAR Cell Products, and a proprietary online system for customizing CAR and CAR cell products, which offers full options to meet all unique needs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION