Background
Mannose-associated serine protease (MASP), also known as mannan-binding lectin serine protease, is an essential enzyme for the activation of the complement lectin pathway. Currently, 3 MASPs have been discovered, namely MASP-1, MASP-2, and MASP-3. MASP-2 is a zymogen encoded by the MASP-2 gene. It is a single-chain polypeptide of 686 amino acid residues, weighing about 76 kDa. Like MASP-1, MASP-2 also contains 6 discrete domains, that are CUB1 (C1r/C1s, urchin-EGF, and bone morphogenetic protein-1) domain, epidermal growth factor (EGF)-like domain, CUB2 domain, two complement control proteins (CCPs), and serine protease domain from N-terminus to C-terminus. Usually, the MASP-2 monomer forms a mature MASP homodimer, resulting from Ca2+-dependent dimerization mediated by the antiparallel CUB1 domain and EGF domain. The two CUB domains are responsible for binding to the collagen-like domains of recognition molecules, and the serine protease domain is the catalytic domain that can cleave substrates.
Among the recognition proteins, MASP-2 can selectively bind to the ficolins and collectins, forming an inactive protein complex. When the complex bind to microbial carbohydrate, zymogen MASP-2 can autoactivate. MASP-1 is essential for its activation under physiological conditions although MASP-2 can autoactivate in vitro. The active MASP-2 cleaves complement C4 and C2 into fragments, generating the C4b2a complex, the C3 convertase in the complement lectin pathway. Besides, MASP-2 is also implicated in the coagulation cascade by cleavage prothrombin. Diseases associated with MASP2 abnormality include MASP-2 Deficiency, complement dysfunctions, and Sporotrichosis.
 Fig. 1 Structure of MASP2.1, 3
Fig. 1 Structure of MASP2.1, 3
MASP2 Functional Service
Creative Biolabs offers a comprehensive suite of MASP2-focused products, such as anti-MASP2 antibodies, ELISA assay kits, and human complement MASP2 proteins. These tools are meticulously crafted and play a crucial role in advancing research aimed at developing therapeutic strategies for various diseases.
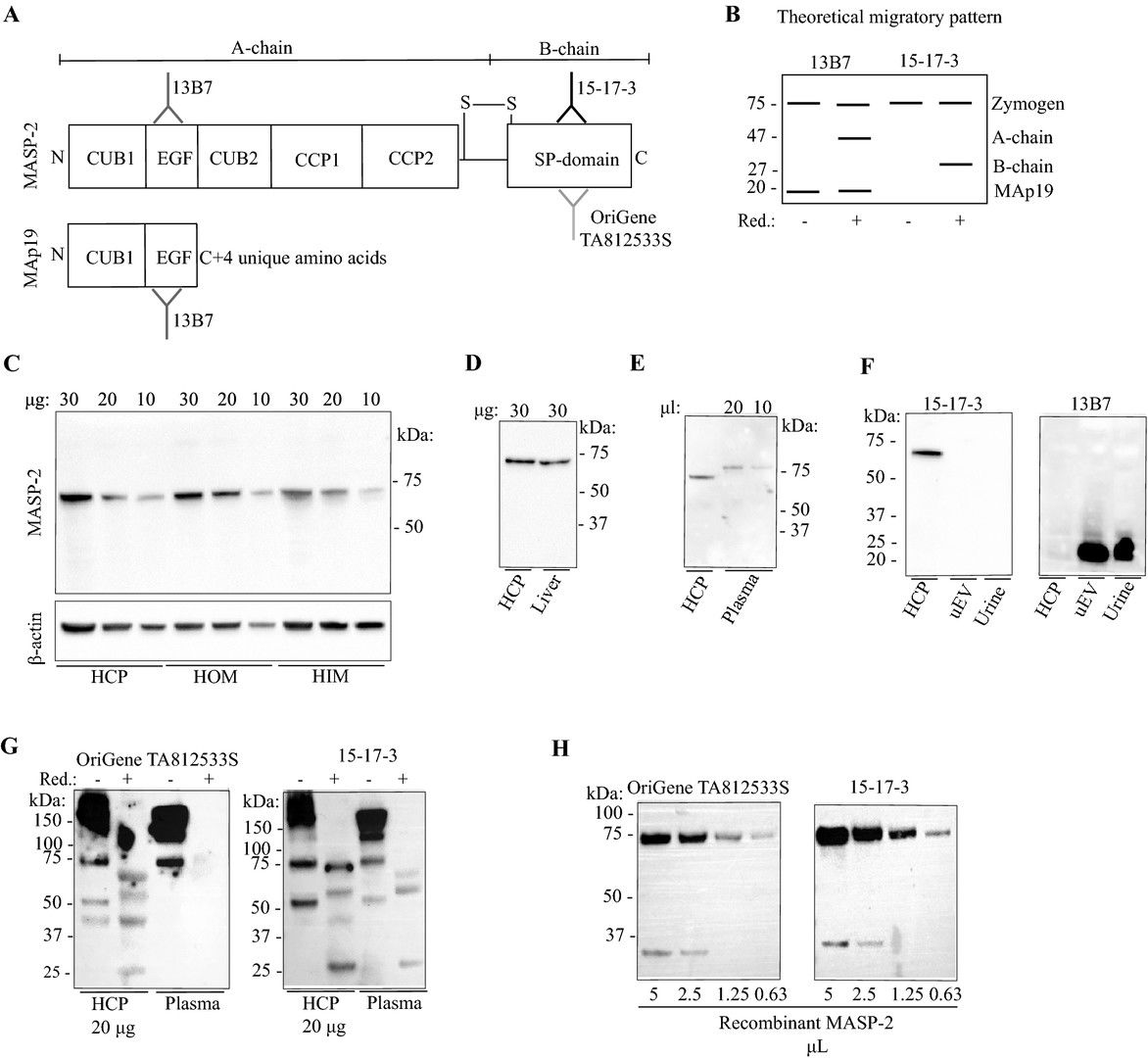 Fig.2 Detection of MASP2 protein isoforms in liver, kidney, and urine via anti-MASP2 antibody.2, 3
Fig.2 Detection of MASP2 protein isoforms in liver, kidney, and urine via anti-MASP2 antibody.2, 3
Aldosterone increases proteolytic activation of the renal epithelial sodium channel (ENaC), yet its sensitive protease remains unidentified. Elevated aldosterone is linked with heightened excretion of MASP-2 in urinary extracellular vesicles. While MASP2 mRNA is highly expressed in the liver, it is absent in kidneys, and aldosterone does not induce its expression in murine or human collecting duct cells. MASP-2 is identified in intercalated kidney cells, indicating protein uptake rather than endogenous expression. Despite dietary sodium variations affecting aldosterone levels, circulating MASP-2 remains stable, and its role in ENaC cleavage is challenged by experimental findings, suggesting MASP-2 is not a physiological activator in this context.
Creative Biolabs delivers an array of specialized MASP2 services, featuring binding assessments and additional functional analyses, specifically crafted to assist our esteemed clients across clinical and research domains.


 Datasheet
Datasheet Fig. 1 Structure of MASP2.1, 3
Fig. 1 Structure of MASP2.1, 3
 Fig.2 Detection of MASP2 protein isoforms in liver, kidney, and urine via anti-MASP2 antibody.2, 3
Fig.2 Detection of MASP2 protein isoforms in liver, kidney, and urine via anti-MASP2 antibody.2, 3
