Discovery and Expression of CRIg
A variety of microbial components, antigen-antibody complexes, and other exogenous or endogenous substances can activate the complement system through three pathways, of which C3 plays a key role in the three pathways. After C3 activation, it can be cleaved into fragments such as C3b, iC3c, and C3dg. These fragments can bind to specific receptors expressed on the surface of different cells and play a role. The complement receptor of the immunoglobulin superfamily (CRIg) is a new C3 receptor that is first reported by Helmy and expressed in Kupffer cells (KC) and is involved in the removal of C3-conditioned pathogenic microorganisms. CRIg is also called V-set and Ig domain (CRIG) / B7 family-related protein.
Human CRIg (huCRIg) has two alternative splicing forms: the long form of human CRIg (huCRIg (L)), which encodes the IgV and IgG2 domains, and the short form of human CRIg (huCRIg (S)), which encodes only the IgV domain . Mouse CRIg (muCRIg) has only one form and encodes the IgV domain. huCRIg (L) is also considered to be Ig superfamily protein 39 (Z39Ig). Studies have found that B cells, T cells, NK cells, and granulocytes in peripheral blood do not express CRIg, and KC in liver expresses CRIg at high levels, while some macrophages have few or no CRIg expression.
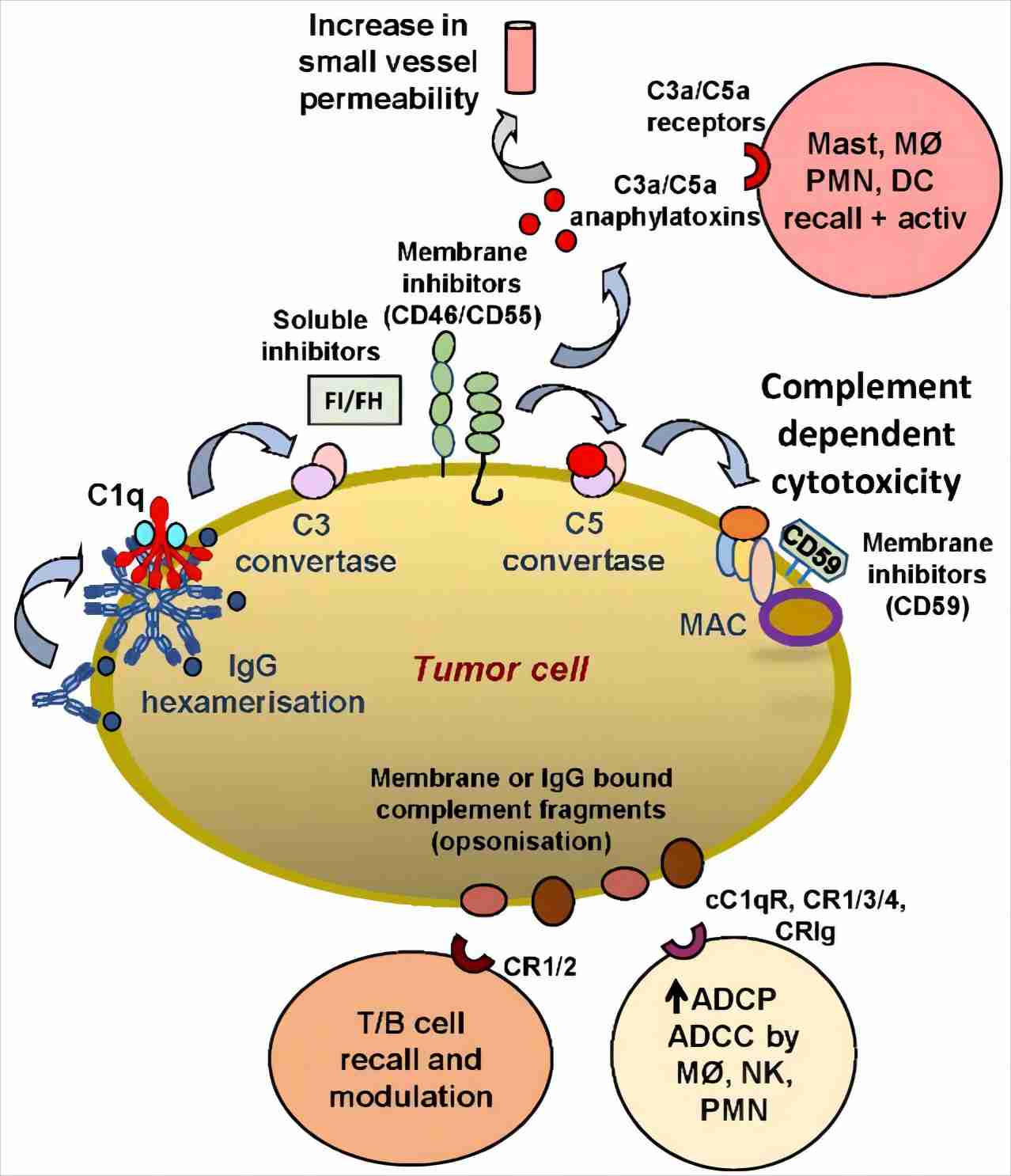
Fig. 1 The role of CRIg on tumor cells.1
Structure of CRIg and Its Interaction with Ligands
The study found that CRIg can bind to IgM-coated sheep red blood cells in the presence of C3. CRIg can also bind to C3b and iC3c-coated culture plates, but cannot bind to other degraded fragments and other complement ligand-coated culture plates. Wiesmann revealed the crystal structure of C3b and C3c when they form a complex with CRIg.
C3b is composed of two chains of α and β. The 645 amino acids of β chain are folded into 5 macroglobulin-like domains (MG1 ~ MG5) and half of MG6 and a linker fragment (LNK). These MG domains form a key Loop-like shape; 746~807 amino acid residues in the α chain and 534~578 amino acid residues in the β chain form the sixth MG domain. The N-terminal domain of CRIg belongs to the IgV immunoglobulin-like domain, and the CRIg domain contains two β sheets.
CRIg binds C3b and C3c in the same way, with the same affinity. The binding site of the CRIg and the TED domain are on both sides of the β chain, and the CRIg on macrophages easily binds to C3b covalently bound to the pathogenic microorganism. The binding site of CRIg is composed of A'B'C'C''F and G chains, and most of them rely on the interaction of amino acid residues on or near the C'C '' chain for binding. MG3, MG4, MG5, MG6 and LNK can all interact with CRIg. Compared with C3, C3b and C3c have their MG3 rotated by 15°, and when CRIg binding sites are formed, the MG3 must be relocated and the LNK region spiral segment must be moved. This is also the reason CRIg can not bind C3.
Functions of CRIg
The function of CRIg is mainly the conditioning effect on pathogenic microorganisms and the inhibitory effect on T cells.
-
Participate in the conditioning of C3-mediated pathogenic microorganisms
The study found that when macrophages encounter C3-conditioned IgM-coated sheep red blood cells, the CRIg will quickly transfer from the transferrin-positive vesicles to the phagocytic cup, lining around the swallowed red blood cells. Further research found that CRIg can actively recruit C3b-coated particles from the circulating endosomes to participate in the formation of early phagosomes. The CRIg is then recycled from the phagosome to the endosome before the phagosome fuses with the lysosome to prevent degradation by the lysosome. Helmy found that the ability of KC of CRIg knockout mice to bind C3-conditioned IgM-coated sheep red blood cells decreased by 60%, indicating that CRIg can be used to clear C3-conditioned pathogen particles in vivo. The key to prevent the occurrence of bacteremia is to quickly capture the pathogenic particles conditioned by C3 in the liver and prevent it from spreading into the blood. In addition, after observing the role of CRIg in clearing intracellular parasitic Listeria monocytogenes (LM) and extracellular bacteria Staphylococcus aureus infection, Helmy concluded that CRIg expressed on KC plays an important role in clearing intracellular or extracellular bacteria of circulatory infection.
-
Suppress T cells
Experiments have proved that CRIg is a negative regulator of T cell response. The extracellular segment of CRIg and IgG1 constant region were fused and expressed in 293-EBNA cells, and the effect of CRIg on T cell proliferation was observed in vitro. It was found that CRIg-Ig can strongly inhibit the proliferation of CD4+ T cells and the production of IL-2. Subsequent experiments found that CRIg-Ig can also inhibit CD8+ T cell proliferation. In terms of effects on T cell function, research has found that CRIg-Ig can reduce the production of antigen-specific CD8+ T cells and IFN-γ, inhibit Th-cell-dependent IgG responses, reduce IgG titers and the number of Qβ-specific B cells and the number of antibody-forming cells.
CRIg also plays an important role in clearing autoantigens such as apoptotic cells and cell debris that bind complement. In addition, researchers also found that CRIg can inhibit the activities of C3 and C5 convertases in the alternative pathway, but does not inhibit the activities of invertases in the classical pathway, which provides a possibility for targeted therapy targeting the complement activation alternative pathway.
Creative Biolabs offers a full range of complement component CRIg-related services. If additional help is needed, please directly contact us and consult our technical supports for more details.
Reference
-
Golay, Josée, and Ronald P. Taylor. "The role of complement in the mechanism of action of therapeutic anti-cancer mAbs." Antibodies 9.4 (2020): 58.
Related Product
For Research Use Only.
Related Sections:

