End-to-End Solution
Featured Services Biomarker Identification Analytical Services Assay Development
Applications Highlights FAQs Resources Related Sections

To make go or no-go decisions at different stages of drug discovery and development, the quality, sensitivity and accuracy of biomarkers are critical to help us make smart decisions. Creative Biolabs is dedicated to biomarker identification, assay development and validation services to provide our clients with the key information that could help move your research forward.
Talk to an Expert →
Our Capabilities
Biomarker analysis and analytical readouts are critical in drug discovery and development, ranging from target validation studies to lead optimization and candidate selection. Hence, the biomarker development to measure the accuracy and sensitivity is necessary for making critical go or no-go decisions. With extensive experience in biomarker identification, assay development and sample processing, Creative Biolabs can support your drug discovery pipeline by providing biomarker development.
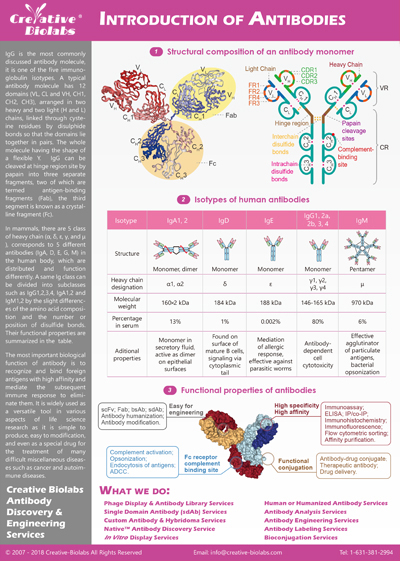
 Fig.1 End-to-End Solution for Biomarker Development.
Fig.1 End-to-End Solution for Biomarker Development.
Services Offered
Biomarker Identification
Identifying novel biomarkers is foundational in understanding disease mechanisms and therapeutic targets. In oncology, biomarkers like VEGF are quantified to predict tumor angiogenesis, leveraging technologies available in tumor marker assays. To identify specific biomarkers for research, we focus on genetics/genomics services using state-of-the-art technologies (such as sequencing, mass spectrometry and real-time PCR) and equipment.
-
Genomics and Proteomics: Cutting-edge technologies such as mass spectrometry and high-throughput sequencing enable the detection of genetic and protein biomarkers.
-
Real-Time PCR: Precise quantification of gene expression levels for targeted biomarker discovery.
-
Digital Biomarkers: Utilizing AI-powered tools for identifying disease-specific molecular patterns.
Analytical Services
Creative Biolabs offers a robust range of analytical services tailored for biomarker development and validation. These services leverage cutting-edge platforms and methodologies such as ELISA, EIA, Western blotting, HPLC, ensuring accuracy, sensitivity, and reproducibility.
|
Category
|
Analytes
|
Primary Platforms
|
|
Neurotransmitters
|
Ach, 5HT, DA, DOPAC, HVA, Glutamate
|
HPLC, Mass Spectrometry
|
|
Growth Factors
|
BDNF, EGF, G-CSF, GM-CSF, IGF-I, VEGF
|
ELISA, Western Blotting, IHC
|
|
Cytokines
|
IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 (p70 or total), IL-13, IL-17A, IL-18, IFN-γ, TNF-α, RANTES, MCP-1, IP-10, Eotaxin, GRO/KC
|
ELISA, Multiplex Immunoassays
|
|
Inflammatory Markers
|
F-kappaB p65, Fractalkine, KC/GRO/CINC (CXCL1), MIP-1α, MIP-2, TGF-β
|
ELISA, Western Blotting, Immunohistochemistry (IHC)
|
|
Metabolic Enzymes
|
LDH Measurement, IDE Activity Assay
|
Enzymatic Activity Assays, HPLC
|
|
Neurodegenerative Markers
|
C99, C83, holoAPP, Aβ1-40, Aβ1-42, Synaptophysin, GAPDH, GLT-1 (GLAST), p-TAU, total TAU
|
Western Blotting, Mass Spectrometry
|
|
Hormones and Proteins
|
EPO, Leptin, Protein Determination Assay
|
ELISA, HPLC
|
|
Other Biomarkers
|
Nitrite Determination, PGE2, P-ser9-GSK3β
|
Spectrophotometry, Mass Spectrometry
|
Assay Development
Creative Biolabs provides extensive assay development services that cater to different stages of biomarker development. The following table reorganizes the capabilities according to the biomarker development stages, ensuring clarity and relevance for researchers.
|
Biomarker Development Stage
|
Assays
|
Capabilities
|
Applications
|
|
Biomarker Discovery
|
Bioanalytical Assays
|
Bioanalytical immunoassays
|
Quantitative analysis of proteins, cytokines, and metabolites to identify potential biomarkers.
|
|
Genetic biomarkers
|
Identification of genetic variations associated with diseases or therapeutic responses.
|
|
Gene expression testing
|
Assessment of mRNA levels to evaluate gene activity and discover novel biomarkers.
|
|
Biomarker Qualification
|
Protein Analysis
|
Protein/phosphoprotein analysis
|
Validation of signaling pathways and post-translational modifications as potential biomarkers.
|
|
Plate-based apoptosis assays
|
Identification of apoptosis markers for drug response validation.
|
|
Sample Resources
|
Biobank resource of flash-frozen samples of our in vivo portfolio available
|
Use of high-quality, well-characterized samples for biomarker qualification.
|
|
Biomarker Validation
|
Tumor Profiling
|
Tumor profiling for target expression and modulation
|
Verification of tumor-specific markers for therapy response and target validation.
|
|
Tumor sampling for in vivo pre-clinical study to monitor for target modulation
|
Validation of therapeutic efficacy through biomarker monitoring.
|
|
Biomarker Implementation
|
Cytokine Analysis
|
Cytokine analysis
|
Profiling inflammatory and immunomodulatory cytokines to establish clinical relevance.
|
|
Biochemical Testing
|
Clinical biochemistry testing
|
Measurement of clinical biomarkers for disease monitoring and therapeutic impact analysis.
|
|
Immunohistochemistry
|
Immunohistochemistry capabilities
|
Localization and quantification of biomarkers in clinical tissue samples for clinical application.
|
Applications
Creative Biolabs' biomarker development services find extensive applications across multiple therapeutic and research domains.
Oncology
Biomarker services play a pivotal role in cancer research, aiding in tumor profiling and the identification of novel therapeutic targets. Biomarkers such as VEGF, IL-6, and p-TAU are essential for monitoring tumor progression, evaluating therapeutic efficacy, and predicting patient responses. These assays support personalized treatment plans by profiling target expression and analyzing tumor modulation in preclinical and clinical studies. For instance, tumor sampling and profiling provide critical data for immuno-oncology therapies, ensuring better-targeted treatment strategies.
Neurology
In neurological research, biomarkers are essential for assessing neurotransmitter activity and detecting neurodegenerative changes. Biomarkers such as Ach, 5HT, and BDNF enable the monitoring of neural pathways, aiding in the development of therapies for conditions like Alzheimer's, Parkinson's, and multiple sclerosis. Services like protein/phosphoprotein analysis and gene expression testing help to map neural networks and evaluate the impact of potential drug candidates.
Immunology and Inflammatory Diseases
Biomarkers such as cytokines (e.g., IL-1, IL-6, TNF-α) are indispensable in studying inflammatory diseases and immune responses. These markers help identify inflammatory pathways and therapeutic targets for diseases such as rheumatoid arthritis, Crohn's disease, and asthma. Creative Biolabs' cytokine analysis and clinical biochemistry testing services support researchers in profiling inflammatory responses and evaluating the efficacy of immunomodulatory therapies.
Metabolic Disorders
Biomarker assays for nitrite, glutamate, and LDH levels are crucial for metabolic research. These markers help in understanding metabolic dysregulation in diseases like diabetes, obesity, and metabolic syndrome. Creative Biolabs provides comprehensive analytical services to quantify these markers and validate their use in therapeutic studies.
Cardiology
In cardiovascular research, biomarkers such as EPO, TGF-β, and fractalkine are instrumental in diagnosing and monitoring conditions like heart failure, atherosclerosis, and myocardial infarction. Creative Biolabs' platforms like bioanalytical immunoassays and protein analysis facilitate the identification of cardiac-specific biomarkers, supporting the development of precision medicine approaches in cardiology.
Infectious Diseases
The identification and validation of biomarkers such as GM-CSF, IFN-γ, and MCP-1 provide insights into host-pathogen interactions and immune responses during infections. These biomarkers are critical in evaluating vaccine efficacy, understanding infection dynamics, and developing novel therapeutics for bacterial, viral, and parasitic diseases.
Why Choose Us?
-
Short turnaround time
-
Comprehensive biomarker database
-
Synergy between scientists across functional areas
-
Rapid GLP validation of developed qualified assays
-
Full solution provider from identification to assay design, measurement, and analytical readouts.
Creative Biolabs offers strategic biomarker assay validation and qualification to help accurately test your samples and provide you with reliable biological translations to make smart decisions in your drug discovery. Whether the assays are performed at your facility or designed and implemented at Creative Biolabs, please feel free to contact us to tailor the best service for you.
FAQs
How do biomarker development services integrate with other offerings?
Our services complement bioassays, immune profiling, and companion diagnostics for a holistic approach to drug discovery.
Can assays be tailored for specific research needs?
Yes, Creative Biolabs offers customized assay development services tailored to specific research goals. From biomarker discovery to validation, the team collaborates with clients to design, develop, and implement assays that address unique study requirements, ensuring optimal results.
What types of biomarkers does Creative Biolabs specialize in?
Creative Biolabs specializes in a wide range of biomarkers, including genetic and protein biomarkers, cytokines, metabolic markers, and immuno-oncology biomarkers. These are analyzed using state-of-the-art platforms to support diverse research and therapeutic applications.
Are digital biomarkers part of Creative Biolabs' offerings?
Yes, Creative Biolabs incorporates AI-powered tools to identify and validate digital biomarkers. These are particularly useful in applications such as neurology and oncology, where advanced molecular pattern recognition enhances biomarker precision and clinical relevance.
Resources
Related Services
Related Products



 Fig.1 End-to-End Solution for Biomarker Development.
Fig.1 End-to-End Solution for Biomarker Development.






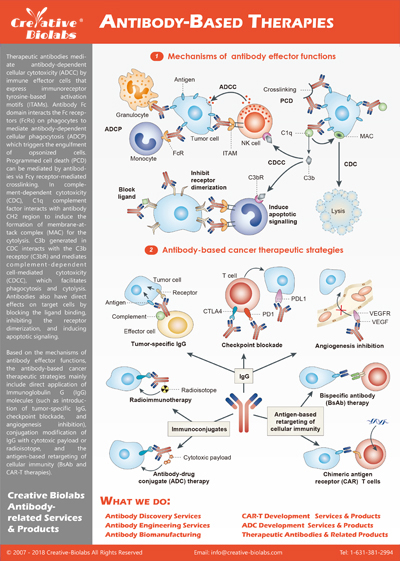

 ADCC, CDC, and ADCP
ADCC, CDC, and ADCP

 Download our brochure
Download our brochure