Creative Biolabs is supported by an advisory board with seasoned professionals in animal modeling, immunology, preclinical research and drug toxicology, analytical chemistry and immunologic analysis. We have accumulated extensive experience from the accomplishment of different projects, during which we have received a lot of recognition and praise from our clients.
Previous studies have demonstrated that the immunogenicity of therapeutic proteins can affect the efficacy of relevant antibodies, leading to high-cost production and serious adverse effects in clinical use. In recent studies, many attempts have been made to assess the immunogenicity of potential drugs during the early phase of discovery. A wide variety of assays have been developed for analyzing the safety of candidate drugs and reducing the production of side effects. Among them, in silico, in vitro, and in vivo models are commonly used to predict the immunogenicity, to reveal immunogenic properties, as well as to decrease the immunogenicity. For instance, in silico models have been generated based on predicting the binding of peptides to MHC molecules. In vitro models are designed to predict the neoepitopes, CD4+ T cell epitopes and the effect on T or B cell activation. Meanwhile, a number of scientists are pioneering studies of “de-immunization methods” to reduce the immunogenicity of therapeutic proteins in animal models. HLA transgenic mouse models have been considered as a perfect model to evaluate the effect of epitope modification on the blocking of HLA binding.
 Fig.1 SIAT® Immunogenicity Assay for Preclinical Study.
Fig.1 SIAT® Immunogenicity Assay for Preclinical Study.
Immunogenicity can be a major issue in the development of successful protein drugs. Pilot studies suggest that anti-drug antibodies (ADAs) caused by immunogenicity may influence the therapeutic function and lead to side effects in patients. As a result, immunogenicity assessment during preclinical development is critical to developing therapeutic proteins with high safety. Creative Biolabs provides a wide collection of SIAT® immunogenicity assays to predict the immunogenicity of therapeutic candidate drugs, including but not limited to:
Creative Biolabs has an entrepreneurial team of highly-skilled and qualified experts in immunology, biochemistry and analytical chemistry. The members have originated from a rich academic research background, bringing their experience into translation. We are very proud of providing high-quality, omnidirectional immunogenicity assay services to our valued clients to remove the difficulties of your projects. If you are interested in our services, please contact us for more details.
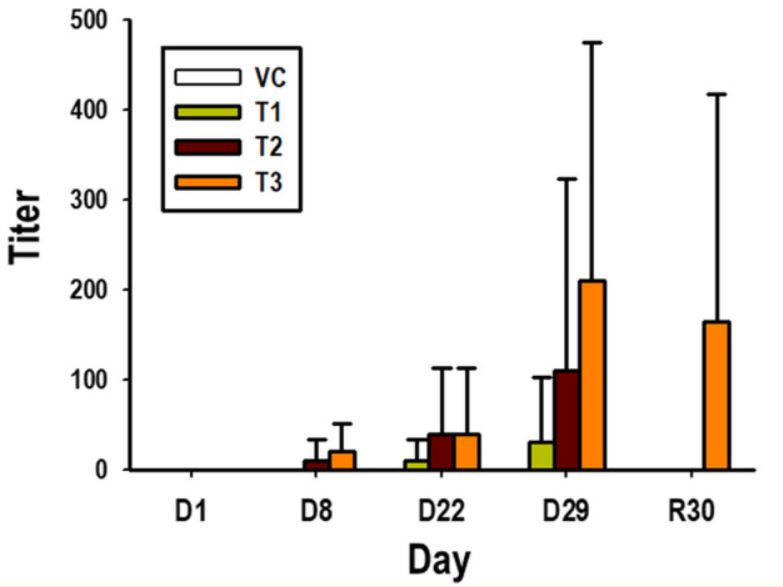 Fig. 2 Measurement of ADAs, representing anti-GX-G3 antibodies, in monkeys subjected to 4-week repeated subcutaneous injection with GX-G3 followed by a 4-week recovery period. (Yun Jung Kim, 2021)
Fig. 2 Measurement of ADAs, representing anti-GX-G3 antibodies, in monkeys subjected to 4-week repeated subcutaneous injection with GX-G3 followed by a 4-week recovery period. (Yun Jung Kim, 2021)
The study focuses on the preclinical immunogenicity testing of GX-G3, an Fc-fused recombinant human granulocyte colony-stimulating factor, using anti-drug antibody (ADA) analysis in rat and monkey models. It highlights the optimized and validated analytical tools used to measure serum levels of GX-G3 and anti-GX-G3 antibodies, determining the immunogenicity responses in these models. The results indicate a lower immunogenicity response in monkeys compared to rats, which correlated with less inhibition of toxicokinetic profiles in monkeys at certain dosages. This research underscores the critical role of ADA assays in evaluating the immunogenicity of biotherapeutics during preclinical studies, providing insights into how ADAs can influence both the safety and efficacy of therapeutic proteins by affecting their pharmacokinetic behaviors.
Immunogenicity assays in preclinical studies are tests used to evaluate the potential immune response against a biologic therapy before it is used in humans. These assays help identify whether the therapeutic protein induces an immune response in animal models, which can predict possible adverse reactions in human clinical trials.
Common animal models used in immunogenicity assays include mice, rats, and non-human primates. The choice of model depends on the similarity of the immune response to humans, the specific disease being treated, and the biological mechanism of the drug.
Doses for immunogenicity testing are selected based on the expected therapeutic dose in humans, scaled to the animal model considering body surface area or metabolic rate. The goal is to evaluate the immune response across a range of doses to understand the dose-response relationship of ADA development.
Interpreting results from preclinical immunogenicity assays can be challenging due to differences in immune systems between animals and humans, variations in ADA detection methods, and the potential for non-relevant ADAs. These factors can complicate the extrapolation of data to predict human responses.
Immunogenicity can significantly impact pharmacokinetics by altering the clearance and distribution of the therapeutic protein. ADAs can form complexes with the drug, increasing clearance or altering its distribution profile, which may lead to reduced efficacy and necessitate dose adjustments.
A bridging ELISA is used in immunogenicity assays to detect ADAs by linking them between drug-coated surfaces and labeled drug probes. This method enhances specificity for the therapeutic drug and is suitable for detecting various ADA isotypes, providing a comprehensive assessment of immunogenicity.
Recent advancements in immunogenicity assays include the development of more sensitive and specific detection methods, the use of computational modeling to predict immunogenicity, and the integration of genomics to understand the genetic basis of immune responses. These improvements help refine risk assessments and enhance the predictive value of preclinical studies.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.