The therapeutic application of proteins in medicine began earnestly in the late 19th century with the application of crude extracts from animal glands. Scientific breakthroughs, such as the discovery of insulin by Banting and Best in the 1920s, signified the dynamic role of proteins in therapy. The success of insulin as a therapeutic protein marked a paradigm shift, paving the way for the subsequent development of recombinant Insulin in 1982. Recombinant therapeutic proteins have become a cornerstone of modern biotechnological therapy, primarily aimed at rectifying defective physiological functions. These proteins are used in treating a spectrum of conditions including cancer, autoimmune diseases, and cardiovascular diseases. With advancements in genetic engineering, there has been a substantial increase in both the diversity and quantity of therapeutic protein drugs.
While therapeutic protein treatment has achieved remarkable success, upon introduction into the body, these proteins trigger immune responses. Being foreign entities to the immune system, therapeutic proteins prompt the production of anti-drug antibodies (ADAs). These antibodies exhibit specific binding to therapeutic proteins, thereby compromising the efficacy of the therapy. The consequences of this immune response can range from mild to severe, depending on its intensity.
Instances of ADA responses have been observed across various types of therapeutic proteins, even when the recombinant protein closely mirrors the endogenous protein. ADAs can lead to a range of clinical outcomes, including diminished drug exposure, reduced treatment efficacy, and potential adverse reactions including hypersensitivity reactions or neutralization of the endogenous protein, possibly resulting in severe diseases or conditions.
The ADA development can be influenced by various factors such as the nature and structure of the therapeutic protein, route and duration of administration, patient-specific variables (such as genetics, age, and immune status), and the presence of impurities. In many instances, the interrelation among these factors significantly influences both the likelihood and severity of ADA development.
Thus, the assessment of immunogenicity in the development of therapeutic proteins is mandatory to identify associated risks and apply proper mitigative measures. This necessitates comprehensive risk assessments, the formulation of effective immunogenicity testing strategies, and the management of immunogenicity related to the product life cycle.
Acknowledging the potential immunogenicity of therapeutic proteins and implementing robust risk assessment strategies is crucial to anticipate and address associated risks effectively. This pivotal measure will further enhance the safety and saturation of therapeutic proteins in the healthcare sector, fostering optimism for a disease-free future.
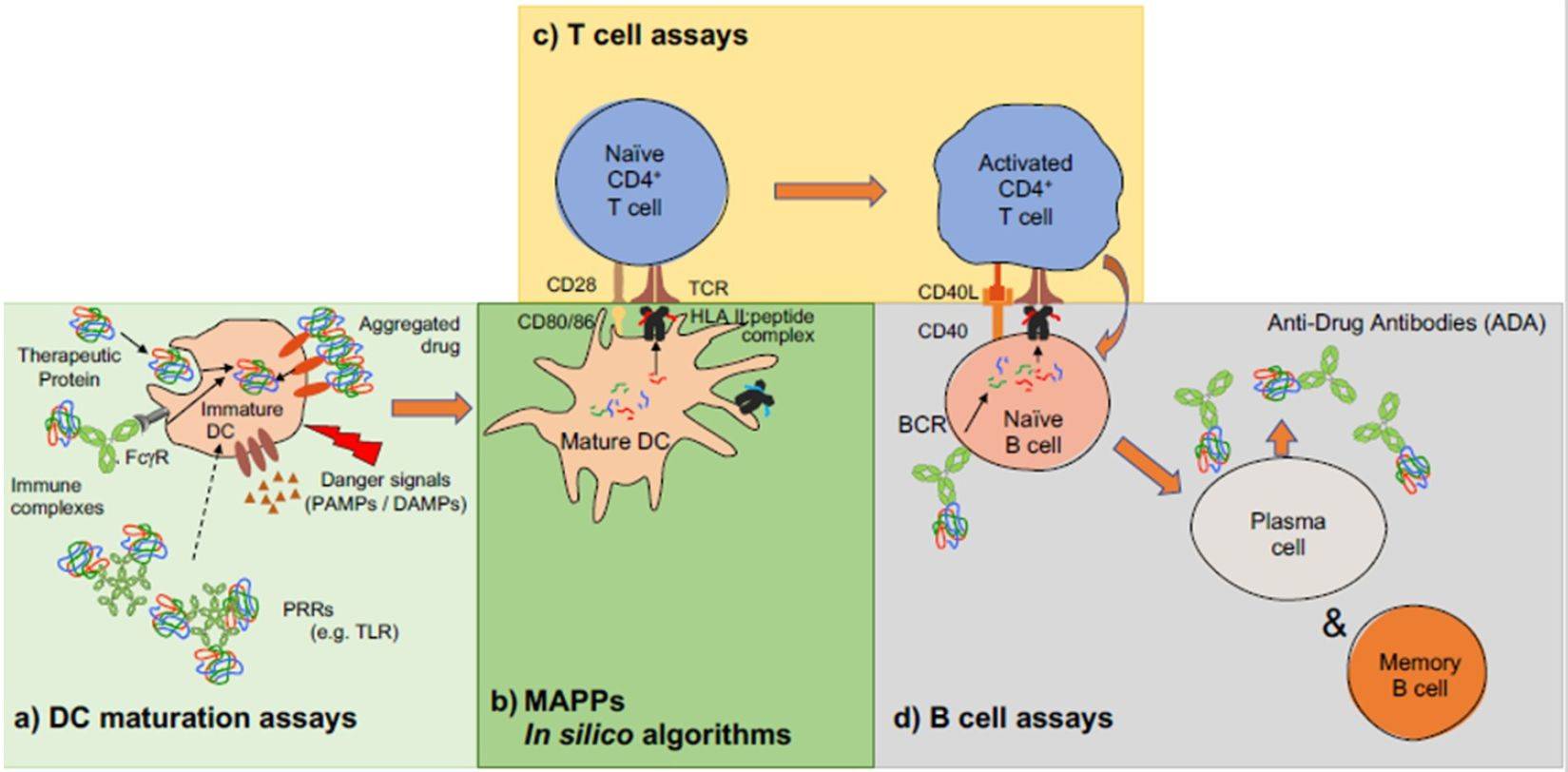 Fig. 1 Schematic overview of an immunogenic signal cascade resulting in the production of anti-drug antibodies. (Ducret Axel, 2022)
Fig. 1 Schematic overview of an immunogenic signal cascade resulting in the production of anti-drug antibodies. (Ducret Axel, 2022)
Immunogenicity risk assessment continues to gain noticeable attention in the field of modern pharmaceutical research. A thorough exploration of this topic yields a deeper comprehension of therapeutic proteins, anti-drug antibodies, and the various factors that impact immunogenicity.
A historical overview offers insight into the emergence of therapeutic proteins as pivotal components of biotechnology. The 1980s witnessed significant growth with the advent of recombinant DNA technology enabling the large-scale production of therapeutic proteins. In the current era of advanced genomics, the healthcare landscape is replete with a diverse array of therapeutic proteins that exert a profound influence on the industry.
Given their biological nature, all therapeutic proteins possess the potential to induce immune responses in patients, leading to immunogenicity. This phenomenon gives rise to the production of anti-drug antibodies (ADAs), molecules engineered by the immune system to neutralize foreign substances. ADAs could signal an undesirable immune reaction, resulting in adverse effects such as diminished the therapeutic efficacy, alterations in pharmacokinetics or pharmacodynamics, and even severe allergic reactions. Consequently, the development of non-immunogenic therapeutic proteins or those with predictable immunogenicity stands as a paramount objective for biomedical researchers.
A closer examination reveals that multiple factors contribute to immunogenicity, broadly categorized as product-related, treatment-related, and patient-related. Product-related considerations encompass the physicochemical properties of the therapeutic protein, level of modification, presence of impurities or contaminants, and the formation of aggregates. Research indicates a direct correlation between protein aggregation and increased immunogenicity in numerous therapeutic proteins.
The dose and route of administration can also influence the degree of immunogenicity. Subcutaneous administration tends to elicit a greater immune response compared to immunogenicity than intravenous administration. Furthermore, long-term treatment is associated with increased immunogenicity and a heightened risk of ADAs.
Patient-specific factors play a significant role in immunogenicity as well. Variations in immune system function due to factors such as age and sex can affect immunogenicity. Additionally, genetic factors unique to each patient contribute to individual differences in immunogenicity.
Emphasis on immunogenicity risk assessment has increased in the last few decades, with regulatory guidelines suggesting the evaluation of all therapeutic proteins for immunogenic risk. By fostering discussions on the significance of therapeutic proteins, anti-drug antibodies, and the factors influencing immunogenicity, researchers, scientists, and medical professionals can deepen their understanding of this complex subject. This knowledge serves as a guiding light for improvement, innovation, and adjustment, ultimately enhancing patient safety and therapeutic efficiency. Future research focused on reducing immunogenicity and improving risk assessment methods provides an exciting trajectory within the dynamic realm of immunogenicity risk assessment.
The field of therapeutic protein development has increasingly recognized the crucial importance of effective tools and methods for conducting robust immunogenicity risk assessment (IRA). The objective of this section is to highlight some pioneering and highly efficient methods adopted in this facet of biotherapeutic development.
These methods underscore the interdisciplinary nature of IRA, emphasizing the need for synergy among diverse tools to establish a comprehensive IRA. It's noteworthy that despite the availability of advanced tools and methodologies, accurately predicting whether a therapeutic protein will stimulate an immune response remains significantly challenging. Consequently, immunogenicity monitoring and risk mitigation should persist throughout the life cycle of the therapeutic protein, extending beyond the approval stage.
As the field of immunogenicity risk assessment continually evolves, the introduction of innovative approaches and strategies will undoubtedly enhance IRA, promising a more promising future for therapeutic proteins.
Creative Biolabs is a leading company in the healthcare sector specializing in offering comprehensive immunogenicity risk assessment services. Creative Biolabs' services include potential immune response prediction, comprehensive evaluation of molecule immunogenicity, and de-immunization strategies. Our ultimate goal is to assist our clients in minimizing the risk of immunogenicity, ensuring the safety and efficacy of their therapeutic products at all times.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
