Anti-drug antibodies (ADAs) represent a response of the body's immune system to pharmaceutical interventions. This immune reaction typically occurs during treatment with biologics, especially in patients undergoing long-term therapy with monoclonal antibodies or protein vaccines.
Biologics consist of large molecular proteins that the body may recognize as foreign substances, thus triggering an immune response that generates antibodies targeting these drugs, known as ADAs. Such response can impact drug efficacy; ADAs may either neutralize the drug's action or expedite its elimination from the body, thereby reducing its duration of action.
In some cases, ADA production can lead to severe adverse effects. For example, in the treatment of severe childhood constipation with albumin-like growth factor, some patients developed ADAs, triggering severe allergic reactions.
Presently, assessing ADA levels has become a widespread clinical practice to evaluate drug safety and efficacy. If ADAs are detected in a patient's bloodstream, treatment adjustments may be necessary, such as switching to alternative medications or modifying dosage and frequency to counteract ADA effects.
As defined by the FDA, immunogenicity refers to the likelihood of a therapeutic protein product causing immune responses against itself, related proteins, or triggering adverse clinical events associated with the immune system. Therefore, when discussing therapeutic proteins, immunogenicity entails undesired immune reactions, which stands in a stark contrast to certain biotech products like vaccines, where an immune response is actually sought.
Numerous factors can influence the immunogenicity of therapeutic proteins, ranging from the origin and characteristics of the proteins themselves. Notably, the immunogenicity varies between fusion, humanized, and fully humanized antibodies. Researchers posit that human antibodies exhibit the lowest immunogenicity, although some exceptions exist where certain human antibodies may still provoke heightened immune responses. Overall, they are considered relatively safe.
Besides the origin of the therapeutic protein, the strength of immunogenicity also correlates with the patient and the type of disease. Patients with autoimmune diseases tend to be more susceptible to the immunogenic effects of therapeutic proteins. The immune system possesses a remarkable ability to discern foreign proteins, even when the disparities are subtle. Moreover, different treatment modalities can influence immunogenicity. Concomitant therapies, for example, are recognized for potentially heightening immunogenicity.
The immune response to biological drugs, particularly the production of ADAs, is a critical aspect of treatment. In the T-cell dependent pathway, monoclonal antibodies (mAbs) serve as antigens and are absorbed by antigen-presenting cells (APCs), where they undergo processing and presentation to T cells through intricate interactions involving MHC class II molecules and the T-cell receptor. ADAs emerge when a T-helper cell distinguishes into either a Th1 or Th2 phenotype, subsequently stimulating B cells to proliferate into plasma cells that produce ADAs, all through a series of complex interactions. Meanwhile, biologics containing multiple epitopes, such as mAbs, can trigger B cell receptors' cross-linking via the T cell-independent pathway, stimulating B cells to directly transform into ADA-producing plasma cells.
Two distinct types of ADAs exist: non-neutralizing on antibodies, also referred to as binding antibodies (Babs), which specifically bind to the drug without interfering with its interaction with the target; and neutralizing ADAs (Nabs), which impede the biological activity of the therapeutic protein by binding to epitopes within or near its active sites or inducing conformational modifications that physically obstruct the drug's ability to bind to its target. Consequently, ADAs possess the potential to modify the pharmacokinetic and pharmacodynamic properties of a drug, thereby diminishing its efficacy. Ultimately, depending on the levels of circulating ADAs, a reduction in drug concentration can have a significant clinical impact. For instance, in patients with low ADA levels, the drug concentration may remain sufficiently high to be effective. However, in patients with elevated ADA levels, a significant portion of the drug will be neutralized, likely leading to a clinical non-response over time.
Non-neutralizing ADAs bind to Fc fragments, interfering with drug action by forming immune complexes with therapeutic proteins. These complexes are more easily cleared by the immune system, reducing drug levels. Non-neutralizing ADAs do not affect the drug's interaction with its target. In contrast, neutralizing ADAs directly affect the drug's binding to the target.
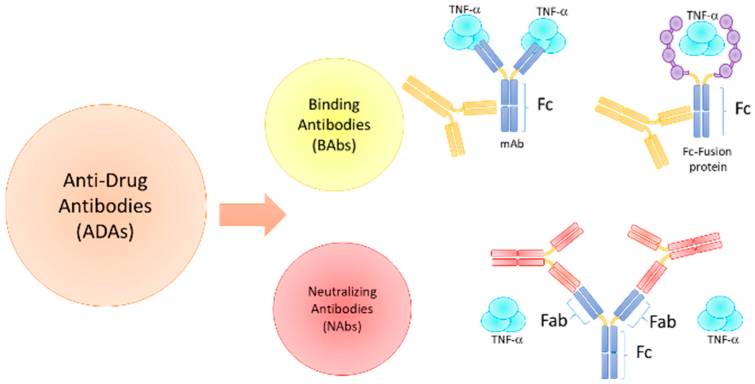 Fig. 1 ADA Types and Modes of Action.1
Fig. 1 ADA Types and Modes of Action.1
It is well known that methods for detecting ADAs must exhibit sensitivity, specificifity, and validation. They should be capable of identifying all types of antibodies against biological agents. Various analytical tests, including ELISA, radioimmune assay (RIA), and electrochemiluminescence (ECL), are employed. However, tracking and quantifying ADAs pose significant challenges, with outcomes varying depending on the method applied.
ADAs can have a significant impact on drug effectiveness and safety, making their detection crucial in the process of drug development and treatment.
Next, several commonly used methods for detecting ADAs are introduced by Creative Biolabs.
In the initial screening for ADAs, ELISA serves as a pivotal method to detect their presence in a patient's blood sample. This technique estimates the quantity ADAs in the sample by measuring the activity of a marker, such as the intensity of the fluorescence signal or the rate of enzyme reaction. The strength of the signal correlates with the presence of drug-resistant antibodies in the sample. In this way, researchers can identify patients who are likely to respond or resist treatment drugs.
While ELISA is effective for initial ADA screening, it may yield false-positive results in some samples. This occurrence may be attributed to the presence of cross-reacting antibodies or other protein fragments in the sample. Hence, all positive results necessitate confirmation tests, such as radioimmunoprecipitation, to ensure result accuracy.
RBA stands as another commonly employed method for ADA detection. This technique uses radiolabelled antigen molecules to identify antibodies in a reaction.
A major advantage of RBA lies in its high sensitivity and specificity, enabling the detection of minute quantities of ADAs. However, compared to alternative testing methods, RBA entails complex operational steps, involves handling radioactive materials, demands sophisticated experimental equipment and skilled personnel, and entails intricate result analysis.
MSD is also widely used in ADA detection, leveraging electrochemical luminescence labeling detected via the MSD platform.
The main steps of this method involve adding a sample (such as blood) to a microplate coated with a drug, where the presence of ADAs in the sample forms an immune complex with the drug. Subsequently, the complex is detected using a chemiluminescent ligin-labeled secondary antibody. In the case of an electric charge, the marker emits light, with the intensity of the glow correlating to the ADA levels in the sample.
The advantage of MSD method is high sensitivity, good repeatability, simple operation, and it can perform multiple tests. However, it is not without limitations; interference may influence results, leading to potential false positives or negatives. Therefore, ADA detection often combines several methods for comprehensive analysis.
SPR offers a direct, real-time, label-free, versatile, rapid, and quantitative technique for analyzing intermolecular interactions. In ADA detection, SPR primarily serves for real-time monitoring and quantification of the binding process between ADAs and drug molecules, providing valuable insights into binding dynamics.
By measuring changes in surface protons generated during the complex formation between drug molecules and ADAs, SPR technology facilitates real-time monitoring of complex formation and deionization rates, thereby yielding binding constants. This information offers critical insights into the strength, affinity and specificity of ADAs.
The SPR detection boasts high sensitivity, eliminates the labeling step, and offers simple operation, facilitating rapid development in ADA confirmation. Despite the complexity of equipment and high detection costs, the technology's capability to provide detailed ADA-drug combination information justifies its use.
Creative Biolabs has developed the SIAT® system for immunogenicity assessment, incorporating a variety of ADA detection methods, including ELISA, SPR, MSD, and RIPA. With comprehensive knowledge, systematic planning, precise data analysis, and extensive experience in immunogenicity assessment, Creative Biolabs stands as a reliable partner in ADA detection and drug development endeavors.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
