Immunogenicity against therapeutic molecules, such as antibody drugs, is a clinical challenge in the successful treatment of many diseases. Therefore, immunogenicity risk assessment in preclinical settings will be useful to improve the efficacy and safety of products-based therapeutics in the development stages. As a first-class provider in the immunologic analysis fields, Creative Biolabs has established Sensitive Immunogenicity Assessment Technology® (SIAT®) system, with attention to in vivo models and assays used to mitigate this risk. Notably, we provide a variety of testing services to therapeutic protein immunogenicity risk assessment, which helps clients harvest more critical information on drug immune safety in the preclinical phase that could be valuable in the success of the drug.
Therapeutic antibodies and proteins may cause an immune response of bodies, leading to the occurrence of anti-drug antibodies (ADAs). Nowadays, therapeutic antibody drugs have significantly promoted the management of numerous chronic diseases and severe disorders. However, their optimal clinical application and further development are complicated by the induction of undesired immune responses. Therapeutic agent-induced ADAs can alter drug pharmacokinetics (PK) and pharmacodynamics (PD) resulting in impaired efficacy and occasionally safety problems. Over the past decade, a growing interest in investigating novel methods to assess the risk of unwanted immunogenicity during preclinical drug development, with the aim to reduce the risk upon the molecular design phase, clinical development, and when drug products reach the market.
The purpose of immunogenicity studies is to characterize an immune response to the product and to identify correlations between ADAs and PK & PD, as well as efficacy & safety. As such, immunogenicity assessment should be contained in the planning of the key clinical trials, including the synchronization of sampling for ADAs and relevant biomarkers, if available, along with the evaluation of efficacy and safety.
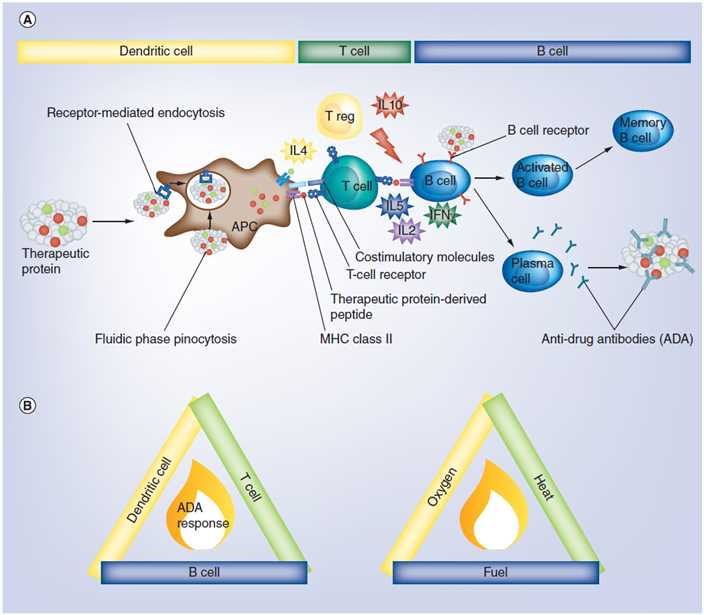 Fig.1 The immunological cascade leading to ADA formation. (Tourdot, 2019)
Fig.1 The immunological cascade leading to ADA formation. (Tourdot, 2019)
Acquiring information on the immunogenic property of a drug prior to clinical trials is pivotal to avoid patient risks and attrition owing to costly drug failure. At Creative Biolabs, we bring a comprehensive meaning to immunogenicity and provide innovative services for preclinical immunogenicity risk assessment of therapeutic antibody drugs. Our current applicable strategies encompass in silico and in vitro methods to understanding the immunological risks associated with interactions of the treatment with crucial classes of immune cells (e.g. T cells, B cells, and antigen-presenting cells).
The planning and evaluation of immunogenicity risk of a biological product have been realized as multidisciplinary exercises. This risk assessment can evolve through the lifecycle of therapeutic protein and may be used to support applications at every step of product development. As an expert in the immune market, Creative Biolabs helps global clients decide the best way to assess immunogenicity of specific biologics using a series of advanced immunogenicity assays. For more information, please directly contact us or discuss with our experts today.
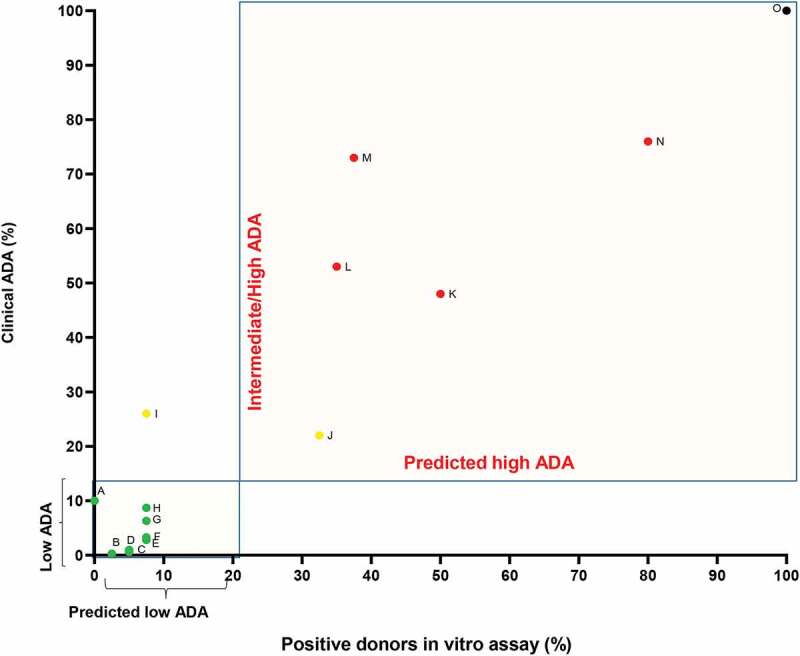 Fig. 2 Summary of clinical ADA frequency and percentage of positive responding donors as determined by in the CD134/CD137 T cell activation assay. (Sivan Cohen, 2021)
Fig. 2 Summary of clinical ADA frequency and percentage of positive responding donors as determined by in the CD134/CD137 T cell activation assay. (Sivan Cohen, 2021)
The study focuses on the immunogenicity risk assessment of biotherapeutics through the development of an in vitro method to measure activation markers CD134 and CD137 on CD4+ T cells. By examining the effects of 14 different biotherapeutics on peripheral blood mononuclear cells from 120 diverse donors, the research identified a correlation between clinical immunogenicity and the proportion of CD4+ T cells expressing CD134 and CD137. Biotherapeutics known for higher rates of anti-drug antibody (ADA) development demonstrated higher levels of these markers. This method provides a quick and effective preclinical test to evaluate the immunogenic potential of new biotherapeutics, enhancing the early screening process to eliminate candidates likely to cause ADA-related adverse effects. This approach is particularly critical in predicting and managing immunogenicity risks before clinical application, ensuring better safety and efficacy outcomes in therapy development.
Immunogenicity risk assessment for therapeutic antibody drugs involves evaluating the potential for these drugs to elicit an undesirable immune response in patients. This assessment is crucial as it helps predict the development of anti-drug antibodies (ADAs) that can neutralize the drug's effects, alter its pharmacokinetics, and potentially lead to adverse clinical outcomes.
It is critical because it informs key decisions throughout the drug development process, from molecular design to clinical trials. Identifying immunogenic risks early allows developers to modify drugs to reduce immunogenic potential, ensuring greater efficacy and safety, and reducing costly late-stage failures.
Immunogenicity is typically assessed using in vitro assays that detect the presence and magnitude of anti-drug antibodies in treated patients. Assays like ELISA, radioimmunoassays, and surface plasmon resonance are used to quantify ADAs, alongside cellular assays that can detect T-cell activation in response to the therapeutic antibody.
While it is challenging to completely eliminate immunogenicity, strategies like humanization of monoclonal antibodies, removal of T-cell epitopes, optimizing glycosylation, and controlling product-related impurities can significantly reduce the immunogenic potential of these therapies.
High immunogenicity can lead to reduced drug efficacy as the immune system neutralizes the therapeutic effect, increased drug clearance, and potentially serious adverse reactions, including infusion reactions and anaphylaxis. It may also necessitate increased dosing or a switch to different therapeutic modalities.
Use the resources in our library to help you understand your options and make critical decisions for your study.
All listed services and products are For Research Use Only. Do Not use in any diagnostic or therapeutic applications.
| USA:
Europe: Germany: |
|
|
Call us at: USA: UK: Germany: |
|
|
Fax:
|
|
| Email: info@creative-biolabs.com |
